Plutonium (III) fluoride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Pu 3+ __ F - | ||||||||||
| Crystal system | ||||||||||
| Space group |
P 3 c 1 (No. 165) |
|||||||||
| Lattice parameters |
a = 709.3 pm |
|||||||||
| Coordination numbers |
Pu [9], F [3] |
|||||||||
| General | ||||||||||
| Surname | Plutonium (III) fluoride | |||||||||
| other names |
Plutonium trifluoride |
|||||||||
| Ratio formula | PuF 3 | |||||||||
| Brief description |
purple crystals |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 301.06 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
9.33 g cm −3 |
|||||||||
| Melting point |
1396 ° C |
|||||||||
| boiling point |
1957 ° C |
|||||||||
| solubility |
almost insoluble in water |
|||||||||
| Hazard and safety information | ||||||||||
 Radioactive |
||||||||||
|
||||||||||
| Thermodynamic properties | ||||||||||
| ΔH f 0 |
−371 ± 3 kcal mol −1 |
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Plutonium (III) fluoride is a chemical compound made up of the elements plutonium and fluorine . It has the formula PuF 3 and belongs to the fluoride class of substances .
presentation
Plutonium (III) fluoride is sparingly soluble and is produced by the reaction of an aqueous plutonium (III) nitrate solution with fluoride salts in acid .
Plutonium (III) fluoride can also be obtained by reacting plutonium (IV) oxalate and hydrogen , plutonium (III) oxalate or plutonium (IV) oxide with hydrogen fluoride .
properties
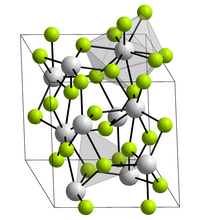
Plutonium (III) fluoride forms violet crystals with a melting point of 1396 ° C. It crystallizes in the lanthanum fluoride structure with the lattice parameters a = 709.3 pm and c = 725.4 pm. Each plutonium core is surrounded by nine fluorine cores in a distorted, triple-capped trigonal-prismatic structure. It is sublimable and has greater volatility than americium (III) fluoride .
use
In order to be able to separate plutonium for reprocessing by precipitation from solutions, a method for precipitation as plutonium (III) fluoride was developed in order to have an alternative to the previous plutonium peroxide method. A 1957 study by Los Alamos National Laboratory reported that this method was less effective than the previous method, while a more recent study commissioned by the US Office of Scientific and Technical Information found this method to be more effective represents.
safety instructions
Classifications according to the CLP regulation are not available, although the chemical toxicity is known. The dangers based on radioactivity are important , provided that the amount of substance involved is relevant.
Individual evidence
- ↑ a b c d e f g Gmelin's Handbook of Inorganic Chemistry , System No. 71, Transurane, Part C, pp. 101-104.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-81.
- ↑ a b Georg Brauer (Ed.): Handbook of Preparative Inorganic Chemistry . 3., reworked. Edition. tape II . Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1299 .
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this substance has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ GA Burney, FW Tober: Precipitation of Plutonium Trifluoride , in: Ind. Eng. Chem. Process Des. Dev. , 1965 , 4 (1), pp. 28-32 ( doi: 10.1021 / i260013a009 ).
- ↑ Stephen C. Carniglia, BB Cunningham: Vapor Pressures of Americium Trifluoride and Plutonium Trifluoride, Heats and Free Energies of Sublimation , in: J. Am. Chem. Soc. , 1955 , 77 (6), pp. 1451-1453 ( doi: 10.1021 / ja01611a015 ).
- ↑ PD Kleinschmidt: Sublimation Studies of Plutonium Trifluoride , in: Journal of Nuclear Materials , 1989 , 167 , pp. 131-134 ( doi: 10.1016 / 0022-3115 (89) 90434-0 ).
- ^ CK Gupta: Hydrometallurgy in Extraction Processes. CRC Press, 1990, ISBN 978-0-8493-6805-9 , pp. 206-208 ( limited preview in Google book search).
- ↑ RS Winchester: Aqueous Decontamination of plutonium from Fission Product Elements . Los Alamos Scientific Laboratory of the University of California, 1957, pp. 9-10 (accessed June 20, 2008).
- ↑ LL Martella, MT Saba, GK Campbell: Laboratory-scale evaluations of alternative plutonium precipitation methods . United States Office of Scientific and Technical Information, 1984 (Retrieved June 20, 2008).
- ↑ KF Grebenkin, Yu. N. Zuev, LN Lokhtin, NA Novoselov, AV Panov, VA Simonenko, VG Subbotin, VM Berezkin, EN Zvonarev, OI Kozlov, VI Lobanov, VP Mashirev, VV Shatalov, AD Maksimov, D. Yu. Chuvilin: Synthesis of Plutonium Trifluoride from Weapons - Plutonium as a Potential Fuel for Power Reactors , in: Atomic Energy , 1997 , 83 (2), pp. 614-621 ( doi: 10.1007 / BF02413891 ).
literature
- David L. Clark, Siegfried S. Hecker, Gordon D. Jarvinen, Mary P. Neu: Plutonium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 813-1264 ( doi : 10.1007 / 1-4020-3598-5_7 ).



