Polymerization
Polymerization (also called polymer formation reaction , according to IUPAC Polymerization ) is a general collective term for synthesis reactions that convert similar or different monomers into polymers . The word polyreaction is occasionally used in German-language literature as a collective term for technical polymerizations .
Technical polymerisation reactions are mostly used for the synthesis of plastics ; they can be divided into chain polymerisation and step growth reactions .
- Chain polymerizations (also called chain growth reactions in English literature ) take place via an active chain end . They can be divided into radical, cationic, anionic and coordinative chain polymerizations.
- Step growth reactions ( called step growth polymerization in English literature ) take place via polycondensation (also called condensation polymerizations ) or polyaddition (also called addition polymerizations ).
Biological polymerisation reactions proceed according to completely different mechanisms and are much more complex, see section Biological polymerisation .
term
Misunderstandings often arise due to inconsistent choice of words and definitions of terms in the German and English specialist literature, as well as the use of terms in the literature that often deviates from the IUPAC proposals . In the German (and especially older) literature, polymerisation often only refers to chain polymerisation . Increasingly, however, the proposal of the IUPAC is followed and the term polymerization is used as a generic term for any polymer formation reaction, as in this article.
The term chain polymerization ( chain polymerization according to IUPAC ) is largely IUPAC-compliant in the German literature - in contrast to the classic word polymerization (see definition of terms ).
The term polyreaction is occasionally used as a synonym for polymerization in the modern (defined here) sense in order to have an alternative to the term used ambiguously.
Classification
Polymer formation reactions generally proceed from low-molecular starting compounds (monomers) to long-chain, often branched, high-molecular molecules ( macromolecules ). In plastics chemistry, the course of growth during polymer formation is important and can be divided into chain growth and step growth.
Chain growth reaction
In chain growth ( chain polymerization ), after a start reaction (initiation), there is a continuous binding of monomers (M) to the growing polymer chain that has arisen from i monomer units (P i ):
- P i + M → P i +1
In this equation i can have all integer values ≥ 2, since no distinction should be made between dimers, oligomers or polymers. Such growth occurs when only the growing polymer chain carries reaction activating functionality. It can be a radical (radical chain polymerization), a cation (cationic chain polymerization), an anion (anionic chain polymerization) or a coordinative complex (coordinative chain polymerization). The formation of the polymers proceeds as an (unbranched) chain reaction . As a result of this growth, macromolecules are formed even at very low conversions and large amounts of monomers are unchanged.
Step growth reaction
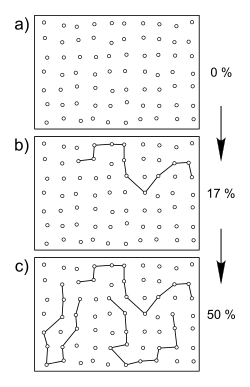
Step growth occurs when the monomers carry at least two functional groups, both of which are independently reactive; they do not proceed as chain growth reactions. During this growth, only dimers, trimers and oligomers are formed at low and medium conversions. High molecular weight products are only formed when the functional groups are almost completely converted. In the following figure, monomers are symbolized as circles.
The green segments of the circles represent two reactive functional groups that all monomers carry at the beginning of the reaction (0% conversion). At 25% conversion, essentially only dimers have formed; the reactive groups have combined to form a chemical bond. Even at 75% conversion, only oligomers are present. In order to form high molecular weight compounds, almost complete conversion is necessary. Step growth can be divided into polyaddition and polycondensation.
Polyaddition
In polyaddition (addition polymerization, IUPAC: polyaddition), growth occurs via addition reactions . First of all, the monomers (M) form dimers (P 2 ) and trimers (P 3 ):
- M + M → P 2
- P 2 + M → P 3
As growth progresses, adducts with any degree of polymerization react with one another. In the reaction equation, i and j can therefore have all integer values ≥ 2.
- P i + P j → P i + j
Polycondensation
Polycondensation (condensation polymerisation, IUPAC: polycondensation) takes place via condensation reactions . Therefore a molecule (L), such as e.g. B. water, split off. The progress of growth is otherwise similar to polyaddition:
- M + M → P 2 + L
- P 2 + M → P 3 + L
- P i + P j → P i + j + L
Chain growth process

The maximum, average degree of polymerization is reached in chain polymerization (1) even with low conversions; the rapid growth is terminated by termination reactions. There is a mixture of the (desired) polymer and many monomers. In the course of the reaction, new, hesitant initiation (propagation) results in the formation of new, again rapidly growing polymer chains, which can lead to complete conversions of the monomers. In the step polymerization (2) only oligomers are formed as the conversion progresses. The maximum, average degree of polymerization is only achieved when the conversion is almost complete. In living polymerization (3), a special case of chain polymerization, the degree of polymerization increases continuously to the maximum, average degree of polymerization, since no termination reactions stop the growth.
Comparison of the growth responses Chain growth reaction Step growth reaction A growing chain only reacts with monomers. Monomers, dimers and oligomers react with one another. The degree of polymerization is almost independent of the conversion. The degree of polymerization depends on the conversion. The reaction mixture contains monomers, macromolecules that no longer grow, and a few growing chains. The mixture contains monomers, oligomers and polymers with different chain lengths. If the conversion is low, there are already polymers with long chains. Polymers with long chains only form at high conversion. An initiator or a special catalyst is necessary to start chain growth. No initiator is required to start a growth reaction. The growth of a chain is terminated by a termination reaction, unless it is a living polymerization. There are no termination reactions.
Biological polymerizations
In living beings , polymerization reactions are used, among other things, for the synthesis of DNA and proteins . They proceed according to different and much more complex mechanisms than those listed above. They usually contain a temporary complex formation with a matrix . In protein biosynthesis, for example, the mRNA serves as a template that forms a complex with ribosomes . The sequence of the template is then transferred to the newly formed polymer. The extremely complex mechanisms allow a high degree of control over the final polymer.
Biological polymerization reactions have so far only been made technically useful in a few cases, for example in the polymerase chain reaction or in the enzymatic polymerization of technical polymers.
Individual evidence
- ↑ a b Entry on polymerization . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04740 Version: 2.3.2.
- ↑ Entry on polyreactions. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ^ MD Lechner, K. Gehrke, EH Nordmeier: Makromolekulare Chemie. 4th edition. Birkhäuser Verlag, 2010, ISBN 978-3-7643-8890-4 , pp. 48-170.
- ^ Wolfgang Kaiser : Synthetic chemistry for engineers. 3rd edition, Carl Hanser, Munich 2011, p. 37.
- ↑ Entry on chain polymerization . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C00958 Version: 2.3.2.
- ↑ Bernd Tieke: Makromolekulare Chemie , 3rd edition, Wiley-VCH, Weinheim, 2014, p. 61.
- ^ Nezha Badi, Jean-François Lutz: Sequence control in polymer synthesis . In: Chemical Society Reviews . 38, No. 12, 2009, p. 3383. doi : 10.1039 / b806413j .
- ↑ J.-F. Lutz, M. Ouchi, DR Liu, M. Sawamoto: Sequence-Controlled Polymers . In: Science . 341, No. 6146, August 8, 2013, pp. 1238149-1238149. doi : 10.1126 / science.1238149 .
- ↑ JohnM.S. Bartlett, David Stirling: A Short History of the Polymerase Chain Reaction . In: Humana Press (Ed.): PCR Protocols . January 1, 2003, pp. 3-6. doi : 10.1385 / 1-59259-384-4: 3 .
- ↑ Shiro Kobayashi, Hiroshi Uyama, Shunsaku Kimura: Enzymatic Polymerization . In: Chemical Reviews . 101, No. 12, December 2001, pp. 3793-3818. doi : 10.1021 / cr990121l .