Polyorthoester
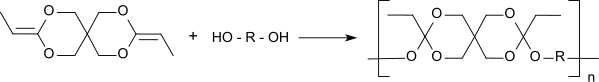
Polyorthoesters are characterized by the general structure - [- R - O - C (R 1 , OR 2 ) - O - R 3 -] n -, where the R 2 radical can also be part of a heterocyclic ring with the R radical. They are produced by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as. B. 3,9-diethylidene-2,4,8,10-tetraoxaspiro [5.5] undecane .
Applications
Polyorthoesters are used as hydrophobic implant materials for drug depots for continuous drug release through surface erosion. surface erosion . The active ingredient homogeneously distributed in a matrix of polyorthoester should be released into the human or animal organism as evenly as possible over a longer period of time with zero order release kinetics. Four classes of polyorthoesters (POE I - IV) are best characterized as biodegradable polymers for drug implants, mainly through the work of Jorge Heller (1927–2009).
presentation
1st generation of polyorthoesters: POE I
The polymer type PEO I is obtained by transesterification of a (usually) α, ω-diol with 2,2-diethoxytetrahydrofuran (from γ- butyrolactone and triethyl orthoformate ).
In transesterification reactions, small molecules are generally split off, in this case ethanol , which must be removed from equilibrium as completely as possible in order to achieve the molar mass of the polymer necessary for handling as an implant material. The solid POE I materials are hydrophobic and particularly sensitive to acids. In an aqueous environment, they suffer uncontrolled autocatalytic hydrolysis and must therefore by adding a basic pharmaceutical excipient (engl. Excipient ) can be stabilized as an implant material. When the polymer chain is broken down, the diol and γ-butyrolactone are formed, which hydrolyze further to γ-hydroxybutyric acid (GHB). GHB is responsible for the local lowering of the pH value during polymer degradation.
The necessary addition of base (e.g. sodium carbonate ), the difficult synthesis and the unsatisfactory mechanical properties have prevented the polyorthoester type POE I from being commercialized.
2nd generation of polyorthoesters: POE II
The polymer type PEO II is produced by polyaddition between an α, ω-diol and the diketene acetal 3,9-diethylidene-2,4,8,10-tetraoxaspiro [5.5] undecane (DETOSU). The polyreaction is much faster than the transesterification to high molar masses and no small molecules are split off as in the case of type POE I. To do this, the monomers are dissolved in tetrahydrofuran and small amounts of an acidic catalyst, e.g. B. p-toluenesulfonic acid added. The molar mass of the polymers can be controlled via the molar ratios of the reactants. The addition of triols leads to crosslinked polymers, the crosslinking density being determined by the triol / diol ratio. The polyreaction takes place quickly even at room temperature and ambient pressure and enables a polymer matrix to be built up in the presence of sensitive pharmaceutically active ingredients.
The solid POE II polymers are very hydrophobic, can be stored in the dry and are significantly less sensitive to acids than type POE I. The pH sensitivity and thus the rate of degradation in a physiological environment, as well as the glass transition temperature of the polymer (and thus the mechanical and thermal properties) can be controlled by the use of diols of different chain flexibility. The resulting polymers of type POE II with molar masses up to over 100,000 therefore have a glass-like-hard consistency ( e.g. when using the rigid diol 1,4-cyclohexanedimethanol ) to semi-soft (when using the more flexible 1,6-hexanediol ). A two-stage, non-autocatalytic polymer hydrolysis takes place in an aqueous medium, which initially produces neutral fragments (pentaerythritol dipropionate and diol).
The propionic acid produced in the second step is metabolized so quickly that it does not lead to a local lowering of the pH value. For this reason, acidic additives such as suberic acid , adipic acid or itaconic acid have to be added to accelerate polymer degradation . With the cytostatic agent , 5-fluorouracil as an embedded drug could release kinetics of almost zero order (engl. Zero-order release ) can be realized. In toxicity tests according to the specifications of the US Pharmacopoeia USP, POE preparations were shown to be acutely non-toxic in cellular, intracutaneous, systemic and intramuscular implants.
3rd generation of polyorthoesters: POE III
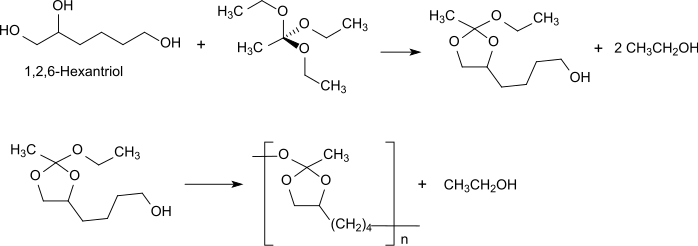
The polymer type POE III, like POE I, is produced by transesterification, in this case a triol, preferably 1,2,6-hexanetriol , with an orthoester such as triethyl orthoacetate (triethyl orthoacetate ).
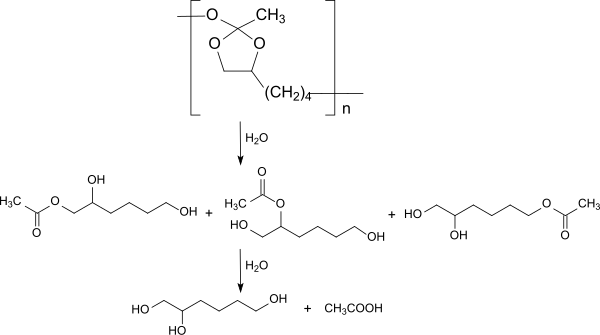
The triethyl orthoacetate initially reacts with the vicinal hydroxyl groups of 1,2,6-hexanetriol to form the corresponding cyclic orthoester, which is transesterified to the polyorthoester type POE III by reaction with the 6-position hydroxyl group. Due to the very flexible polymer backbone, polyorthoesters of the POE III type are semi-solid to ointment-like at room temperature and allow thermolabile and solvent-sensitive active ingredients to be incorporated at room temperature without organic solvents. The active substance implants obtained are particularly suitable for applications on the eye, with no sudden release of active substance through diffusion. initial burst release , but a continuous release following the polymer degradation. In POE III, too, polymer degradation occurs through cleavage of the hydrolytically labile bonds in the polymer backbone in the sense of surface erosion.
Depending on the initial bond cleavage at the quaternary carbon atom, 1-, 2- or 6-acetoxy-hexanetriol are formed, which are further degraded to 1,2,6-hexanetriol and acetic acid . The lengthy synthesis to build up polymers with useful molecular weights and the poor reproducibility limit the use of the type POE III for biomedical applications.
4th generation of polyorthoesters: POE IV
The polymer type POE IV is a further development of the type POE II, in which the diketene acetal DETOSU is combined with a diol that has short sequences of polyglycolide or polylactide . Depending on the type of diol used, POE IV can be synthesized as a gel (with a low glass transition temperature T g , i.e. low molar mass) or as a solid. POE IV types are also accessible under the very mild conditions of interfacial polycondensation.
POE IV avoids the addition of acidic excipients required for POE II, which often diffuse out of the polymer matrix in an uncontrolled manner and thus lead to erratic degradation kinetics. When polyorthoesters of the type POE IV break down in aqueous media, glycolic acid or lactic acid is formed , which catalyze further hydrolysis.
The rate of degradation can be controlled by the proportion of glycol or lactic acid sequences. Implants made of POE IV show surface erosion with high biocompatibility with degradation times between days and months and can therefore also be used as long-term active ingredient depots, e.g. B. used for the cytostatic 5-fluorouracil.
Polyorthoesters of type POE IV are considered to be the most promising representatives of this class of substances as implant materials for the controlled release of active substances.
literature
- M. Chasin, R. Langer (Eds.): Biodegradable Polymers as Drug Delivery Systems, in Drugs and the Pharmaceutical Sciences . tape 45 . Marcel Dekker, Inc., 1990, ISBN 0-8247-8344-1 .
- KE Uhrich, SM Cannizzaro, RS Langer, KM Shakesheff: Polymeric Systems for Controlled Drug Release . In: Chem. Rev. Band 99 , no. 11 , October 26, 1999, p. 3181-3198 , doi : 10.1021 / cr940351u .
- J. Heller: Biopolymers I: Poly (ortho esters) . In: Advances in Polymer Science . tape 107 , June 11, 2005, p. 41-92 , doi : 10.1007 / BFb0027551 .
- Ray Smith (Ed.): Biodegradable Polymers for Industrial Applications . tape 45 . CRC Press, 2005, ISBN 0-8493-3466-7 .
- JH Park, M. Ye, K. Park: Biodegradable Polymers for Microencapsulation of Drugs . In: Macromolecules . tape 10 , 2005, pp. 146–161 ( mdpi.org [PDF]).
- BD Ratner, AS Hoffman, FJ Schoen, JE Lemons (Eds.): Biomaterials Science: An Introduction to Materials in Medicine . 3. Edition. Academic Press, 2013, ISBN 978-0-12-374626-9 .
Individual evidence
- ↑ a b N.N .: Polymers as biomaterials. (online at: usm.edu )
- ↑ J. Heller, KJ Himmelstein: Poly (ortho ester) biodegradable polymer systems . In: Methods Enzymol. tape 112 , 1985, pp. 422-436 , doi : 10.1016 / Soo76-6879 (85) 12033-1 .
- ^ Controlledreleasesociety.org
- ↑ Patent US4990631 : Reacting trialkyl orthoformate with boron trifluoride and lactone, then with alkoxide or alkanol and base. Published February 5, 1991 , Applicant: Alza Corp., Inventor: K. Alster.
- ↑ J. Heller: Poly (Ortho Esters) . In: A. Lendlein, A. Sisson (Eds.): Handbook of Biodegradable Polymers: Synthesis, Characterization and Applications . Wiley-VCH, 2011, ISBN 978-3-527-32441-5 .
- ↑ a b Jorge Heller, John Barr, Steven Y. Ng, Khadija Schwach Abdellauoi, Robert Gurny: Polyanhydrides and Poly (ortho esters): Poly (ortho esters): synthesis, characterization, properties and uses . In: Advanced Drug Delivery Reviews . tape 54 , no. 7 , October 16, 2002, p. 1015-1039 , doi : 10.1016 / S0169-409X (02) 00055-8 .
- ↑ M. Bhattacharya, RL Reis, V. Corello, L. Boesel: 13. Material properties of biodegradable polymers . In: CRC Press . tape 16 , no. 1–2 (June – July), 1991, pp. 3-13 , doi : 10.1016 / 0168-3659 (91) 90026-A .
- ↑ J. Heller, YF Maa, P. Wuthrich, R. Duncan: Recent developments in the synthesis and utilization of poly (ortho esters) . In: J. Controlled Release . tape 16 , no. 1–2 (June – July), 1991, pp. 3-13 , doi : 10.1016 / 0168-3659 (91) 90026-A .
- ^ AU Daniels, KP Andriano, WP Smutz, MKO Chang, J. Heller: Evaluation of absorbable poly (ortho esters) for use in surgical implants . In: J. Appl. Biomaterials . tape 5 , no. 1 , August 30, 2005, p. 51-64 , doi : 10.1002 / jab.770050108 .
- Jump up ↑ S. Einmahl, M. Zignani, E. Varesio, J. Heller, JL Veuthey, C. Tabatabay, R. Gurny: Concomitant and controlled release of dexamethasone and 5-fluorouracil from poly (ortho ester) . In: Int. J. Pharm. Volume 185 , no. 2 , August 20, 1999, p. 189-198 , doi : 10.1016 / S0378-5173 (99) 00149-0 .
- ^ S. Einmahl, F. Behar-Cohen, F. D'Hermies, S. Rudaz, C. Tabatabay, R. Gurny: A New Poly (Ortho Ester) -Based Drug Delivery System as an Adjunct Treatment in Filtering Surgery . In: IOVS . 42 number = 3, March 2001, p. 695-700 .
- ^ SY Ng, T. Vandamme, MS Taylor, J. Heller: Synthesis and erosion studies of self-catalyzed poly (ortho ester) s . In: Macromolecules . tape 30 , no. 4 , February 24, 1997, p. 770-772 , doi : 10.1021 / ma9610626 .
- ↑ J. Heller, J. Barr: Poly (ortho esters) - from concept to reality . In: Biomacromolecules . tape 5 , no. 5 , August 17, 2004, p. 1625-1632 , doi : 10.1021 / bm040049n .
- ↑ K. Bonchemal, S. Briancon, P. Chaumont, H. Fessi, N. Zydowicz: Microencapsulation of dehydroepiandrosterone (DHEA) with poly (ortho ester) polymers by interfacial polycondensation . In: J. Microencapsulation . tape 20 , no. 5 , 2003, p. 637-651 , doi : 10.1080 / 0265204031000148040 .
- ^ SY Ng, HR Shen, E. Lopez, Y. Zherebin, J. Barr, E. Schacht, J. Heller: Development of a poly (ortho ester) prototype with a latent acid in the polymer backbone for 5-fluorouracil delivery . In: J. Control Release . tape 65 , no. 3 , 2000, pp. 367-374 , doi : 10.1016 / S0168-3659 (99) 00218-7 .
- ↑ J. Heller, J. Barr: Poly (ortho esters): Some recent developments, in Polymeric Drug Delivery II . In: ACS Symposium Series . tape 924 , March 9, 2006, chap. 3 , p. 29-43 , doi : 10.1021 / bk-2006-0924.ch003 .