3,9-diethylidene-2,4,8,10-tetraoxaspiro (5.5) undecane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3,9-diethylidene-2,4,8,10-tetraoxaspiro [5.5] undecane | |||||||||||||||
| other names |
DETOSU |
|||||||||||||||
| Molecular formula | C 11 H 16 O 4 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 212.24 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
30 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
Insoluble in water, soluble in pentane , in n -hexane , in heptane , in tetrahydrofuran and 1,4-dioxane |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
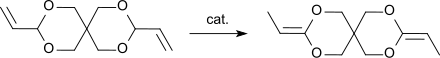
3,9-Diethylidene-2,4,8,10-tetraoxaspiro [5.5] undecane , DETOSU for short , is a bicyclic ketene acetal which is different from the isomeric allylacetal 3,9-divinyl-2,4,8,10-tetraoxaspiro [5.5 ] undecan , DVTOSU for short. As a bifunctional monomer, DETOSU is an important building block for polyorthoesters , which are formed by the addition of diols to the activated double bond of the diketene acetal.
presentation
The rearrangement of the DVTOSU into the DETOSU is an exothermic reaction which also takes place spontaneously with complete conversion. For production on an industrial scale, DVTOSU is rearranged at elevated temperatures in the presence of catalysts.
In addition to carrying out the rearrangement reaction in an alkaline medium, such as. B. with n-butyllithium in ethylenediamine or potassium tert-butoxide in ethylenediamine, the reaction can also be carried out photochemically by UV irradiation in the presence of iron pentacarbonyl as a catalyst and triethylamine in boiling pentane or with tris (triphenylphosphine) ruthenium (II) dichloride - sodium carbonate in Substance to be carried out.
In order to obtain usable purities for use as a monomer, the crude product obtained after the rearrangement reaction and vacuum distillation has to be recrystallized several times from pentane. The yields of pure product are around 50% of theory.
properties
3,9-Diethylidene-2,4,8,10-tetraoxaspiro [5.5] undecane is a material which is crystalline at room temperature in the pure state. Because of its low tendency to crystallize, it is mostly used as a liquid. DETOSU is relatively unstable. Even in the presence of traces of water, it hydrolyzes rapidly and isomerizes spontaneously during storage to the diallylacetal DVTOSU, which is inactive for the polyreaction. The pure substance is very reactive to attack by electrophilic agents and has a strong tendency towards cationic polymerization. DETOSU is characterized by the intense IR band at 1700 cm −1 , which can also be used to follow the conversion during the rearrangement reaction.
use
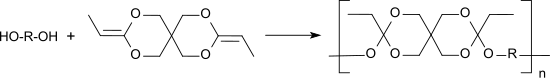
The diketene acetal 3,9-diethylidene-2,4,8,10-tetraoxaspiro [5.5] undecane, DETOSU, is a reactive bifunctional monomer that forms biodegradable polyorthoesters by polyaddition with α, ω-diols.
Polyorthoesters are used as embedding media for pharmaceuticals in depot pharmaceutical forms for the controlled release of active ingredients by surface erosion under physiological conditions.
Individual evidence
- ↑ a b c d e f g h i Patent US5939453 : PEG-POE, PEG-POE-PEG, and POE-PEG-POE block copolymers. Published August 17, 1999 Applicant: Advanced Polymer Systems, Inc. Inventor: J. Heller, SY Ng.
- ↑ a b c d Patent US6863782 : Method of preparing di (ketene acetals). Published on March 8, 2005 , Applicant: AP Pharma, Inc., inventors PW Newsome et al
- ↑ a b c J. V. Crivello, R. Malik, Y.-L. Lai ,: Ketene acetal monomers: Synthesis and characterization . In: J. Polym. Sci. A polym. Chem. Band 34 , 1996, pp. 3091-3102 , doi : 10.1002 / (SICI) 1099-0518 (19961115) 34:15 <3091 :: AID-POLA1> 3.0.CO; 2-0 .
- ↑ a b c Patent US4532335 : Preparation of ketene acetals by rearrangement of allyl and substituted allyl acetals. Published July 30, 1985 , Applicant: SRI International, Inventor: RF Helwing.
- ↑ a b K. Bouchemal, S. Briancon, P. Chaumont, H. Fessi, N. Zydowicz: Microencapsulation of dehydroepiandrosterone (DHEA) with poly (ortho ester) polymers by interfacial polycondensation . In: J. Microencapsulation . tape 20 , no. 5 , 2003, p. 637-651 , doi : 10.1080 / 0265204031000148040 .
- ↑ Patent US4549010 : Bioerodible poly (ortho ester) thermoplastic elastomer from diketenen diacetal. Filed June 27, 1984 , published October 22, 1985 , Applicant: Merck & Co., Inc., Inventor: RV Sparer, SA Pogany.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ J. Heller, KJ Himmelstein: Poly (ortho ester) biodegradable polymer systems . In: Methods in enzymology . tape 112 , 1985, pp. 422-436 .
- ↑ E. Piskin: Biodegradable Polymers in Medicine . In: G. Scott (Ed.): Degradable Polymers: Principles and Applications . 2nd Edition. Kluwer Academic Press, 2002, ISBN 1-4020-0790-6 .
- ↑ J. Heller: Poly (Ortho Esters) . In: A. Lendlein, A. Sisson (Eds.): Handbook of Biodegradable Polymers: Synthesis, Characterization and Applications . Wiley-VCH, 2011, ISBN 978-3-527-32441-5 .
- ↑ J. Heller: Poly (ortho esters) . In: Robert S. Langer, Nicholas A. Peppas (Eds.): Biopolymers I. Advances in Polymer Science . Springer, Berlin / Heidelberg 1993, ISBN 3-540-56148-X , p. 41-92 .
- ^ J. Heller: Development of poly (ortho esters): a historical overview . In: Biomaterials . tape 11 , no. 9 , 1990, pp. 659-665 , doi : 10.1016 / 0142-9612 (90) 90024-K .