Ponatinib
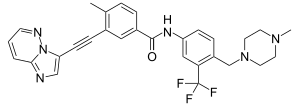
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Ponatinib | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 29 H 27 F 3 N 6 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 532.22 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ponatinib (trade name Iclusig ; manufacturer Ariad Pharmaceuticals ) is a drug that is used in the treatment of chronic myeloid leukemia (CML) or BCR -ABL -positive acute lymphoblastic leukemia (Ph + ALL).
Mechanism of action
Ponatinib is a tyrosine kinase inhibitor (TKI) with antiangiogenic and antineoplastic effects. The orally applicable substance inhibits various tyrosine kinases , including those from the ABL , SRC , VEGFR and FGFR families.
Ponatinib is of great therapeutic interest because the substance is also effective against the BCR-ABL -T315I mutation, for which all other previously approved TKIs ( imatinib , nilotinib , dasatinib , bosutinib ) are ineffective. The substance is effective in doses of 45 mg once a day.
Development history
Ponatinib was developed by the pharmaceutical company Ariad Pharmaceuticals , initially under the internal name 'AP24534'. The application for accelerated approval submitted to the American Food and Drug Administration (FDA) in August 2012 was approved in December 2012. Approval was granted for the treatment of patients with chronic myeloid leukemia (CML) or BCR-ABL- positive acute lymphoblastic leukemia (ALL) who are resistant to other tyrosine kinase inhibitors or to whom these cannot be administered due to intolerance . The main basis was the results of the PACE study. The drug was marketed in the United States under the trade name Iclusig . In July 2013, approval for the same areas of application by the EU Commission for the European market followed.
The updated results of the PACE study, published in November 2013 in the New England Journal of Medicine , showed positive ALL in a group of 449 intensively pretreated patients with CML or BCR-ABL- positive ALL in whom nilotinib and dasatinib were either not tolerated or ineffective carried the BCR-ABL T315 mutation, an overall remarkable potency. The effect, however, was significantly worse with CML in blast crisis and ALL than with CML in the chronic phase. Arterial thrombotic events were observed in 9% of ponatinib-treated patients, of which 3% were attributed to treatment.
As part of the EPIC study ( E valuation of P onatinib versus I matinib in C hronic Myeloid Leukemia) the efficacy of ponatinib compared to was imatinib in previously untreated CML patients, d. H. checked in the first line . On October 18, 2013, Ariad Pharmaceuticals announced the premature discontinuation of this study after an increased incidence of arterial thrombosis in ponatinib-treated patients. The company announced that it would not include any new patients in ongoing clinical trials until new dosage recommendations (possibly 30 mg or 15 mg instead of 45 mg) and safety measures for use, such as simultaneous administration of anticoagulants , had been worked out in cooperation with the FDA .
Side effects
The most common side effects, including serious ones, observed in more than 1% of those treated were skin changes (rash, dryness), abdominal pain, fever, anemia, changes in the blood count ( neutropenia , thrombocytopenia , pancytopenia ), inflammation of the pancreas ( pancreatitis ), heart attack , diarrhea and elevated lipase levels.
In the long-term follow-up of patients in ongoing Phase 1 and Phase 2 studies, an increase in arterial ( cardiovascular , cerebrovascular and peripheral vascular) and venous thrombotic events was observed, leading to the introduction of appropriate restrictions and safety measures for patients with cardiovascular risk factors.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Dave Levitan: Accelerated FDA Approval for CML Drug Ponatinib Sought. (No longer available online.) CancerNetwork, Aug 9, 2012, archived from the original on Nov 20, 2012 ; accessed on December 25, 2012 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b ponatinib. December 17, 2012, accessed December 25, 2012 .
- ↑ Jorge E. Cortes, Hagop Kantarjian, Neil P. Shah, Dale Bixby, Michael J. Mauro, Ian Flinn, Thomas O'Hare, Simin Hu, Narayana I. Narasimhan, Victor M. Rivera, Tim Clackson, Christopher D. Turner , Frank G. Haluska, Brian J. Druker, Michael WN Deininger, Moshe Talpaz: Ponatinib in refractory Philadelphia chromosome-positive leukemias . In: The New England Journal of Medicine . tape 367 , no. 22 , November 29, 2012, p. 2075-2088 , doi : 10.1056 / NEJMoa1205127 , PMID 23190221 .
- ↑ ARIAD announces approval of Iclusig® (ponatinib) in the European Union. PharmaZeitung.de, July 2, 2013, accessed on July 4, 2013 .
- ↑ JE Cortes, D.-W. Kim, J. Pinilla-Ibarz, P. le Coutre, R. Paquette, C. Chuah, FE Nicolini, JF Apperley, HJ Khoury, M. Talpaz, J. DiPersio, DJ DeAngelo, E. Abruzzese, D. Rea, M Baccarani, MC Müller, C. Gambacorti-Passerini, S. Wong, S. Lustgarten, VM Rivera, T. Clackson, CD Turner, FG Haluska, F. Guilhot, MW Deininger, A. Hochhaus, T. Hughes, JM Goldman , NP Shah, H. Kantarjian, PACE Investigators: A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias . In: The New England Journal of Medicine . tape 369 , no. 19 , November 7, 2013, pp. 1783-1796 , doi : 10.1056 / NEJMoa1306494 , PMID 24180494 .
- ↑ Ponatinib in Newly Diagnosed Chronic Myeloid Leukemia (CML) (EPIC). clinicaltrials.gov, accessed January 15, 2013 .
- ↑ ARIAD Announces Discontinuation of the Phase 3 Trial of Epic Iclusig in Patients with Newly Diagnosed Chronic Myeloid Leukemia. (No longer available online.) Investor.ariad.com, formerly in the original ; accessed on October 21, 2013 (English). ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ ARIAD Announces Changes in the Clinical Development Program of Iclusig. (No longer available online.) In: ARIAD Pharmaceuticals. October 8, 2013, archived from the original on June 22, 2014 ; accessed on October 21, 2013 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Product information on Iclusig (PDF) from the European Medicines Agency (EMA).
- ↑ Rote Hand Brief from ARIAD Pharmaceuticals GmbH in December 2013. (PDF; 78 kB) Retrieved on December 3, 2013 .