Riociguat
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
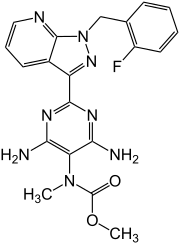
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Riociguat | |||||||||||||||||||||
| other names |
Methyl- N - [4,6-diamino-2- [1 - [(2-fluorophenyl) methyl] -1 H -pyrazolo [3,4- b ] pyridin-3-yl] -5-pyrimidinyl] - N - methyl carbaminate ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 20 H 19 FN 8 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| Drug class |
sGC stimulators |
|||||||||||||||||||||
| Mechanism of action |
Stimulation of soluble guanylate cyclase |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 422.415 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Riociguat is a medicine that can be used to treat chronic thromboembolic pulmonary hypertension ( CTEPH ) and certain types of pulmonary artery hypertension ( PAH ). Riociguat is a stimulator of soluble guanylate cyclase (sGC), an important enzyme in the nitric oxide signaling pathway. Riociguat was developed by Bayer .
Discovery and development
The first nitric oxide-independent, heme-dependent guanylate cyclase stimulator YC-1, a synthetic benzylindazole derivative, was described in 1978. The pharmacological characterization of this substance 20 years later showed that YC-1 not only increased the activity of soluble guanylate cyclase, but also synergistically stimulated guanylate cyclase with NO. However, YC-1 was a relatively weak vasodilator and also showed side effects. The search among the indazole active ingredients resulted in the identification of BAY 41-2272 and BAY 41-8543. The results showed an improvement in the systemic arterial oxygen supply. Further tests led to the discovery of riociguat. In the animal model, the drug effectively reduced pulmonary hypertension and disease-related hypertrophy of the right heart with further structural changes in both ventricles.
In 2013, the riociguat was approved as a drug in the USA. In 2014, the approval recommendation was made by the European Medicines Agency EMA.
Chemistry and mode of action
Nitric oxide (NO) acts as a signaling molecule in smooth muscle cells to trigger blood vessel dilation. In patients with pulmonary arterial hypertension (PAH), the enzyme NO synthase, which causes NO production, is only present to a reduced extent. As a result, lower concentrations of cellularly produced NO and vasoconstriction are found in these patients . NO binds as a signaling molecule to the enzyme soluble guanylate cyclase (sGC) and thus induces the production of the secondary signaling molecule ( second messenger ) cyclic guanosine monophosphate (cGMP). The synthesized cGMP activates the cGMP-dependent protein kinase ( protein kinase G ), which regulates the cytosolic calcium ion concentration. The change in the calcium level in the cell then causes the blood vessel to dilate via the change in the actin - myosin contraction. In the presence of PAH, this path is disturbed because there is not enough NO available or it has an insufficient effect. In contrast to NO or heme-independent sGC activators, e.g. B. cinaciguat, riociguat acts as a direct sGC stimulator independent of NO. Furthermore, riociguat works synergistically with NO and achieves anti-aggregatory, anti-proliferative and vasodilatory effects.
pharmacology
Riociguat increases the activity of the enzyme sGC in concentrations between 0.1 and 100 mmol / l. Riociguat also works in synergy with the NO donor complex diethylamine / NO and can increase sGC activity in vitro. A phase I clinical study showed that riociguat is rapidly absorbed by the body and that the maximum plasma concentration is reached after approximately 0.5–1.5 hours. The average elimination half-life is between 5 and 10 hours. Riociguat plasma concentrations can be relatively variable in different patients, which is why the dose at the start of therapy is individually tailored to the patient (titration).
Clinical studies
Various clinical studies have been and are being carried out to investigate various aspects of riociguat.
The first study to confirm the concept of action of riociguat in patients with PAH was carried out in the Lung Center of the University of Giessen . To this end, nineteen patients were evaluated. The drug was well tolerated and was longer effective and more efficient when compared to NO. Other studies were CHEST-1 and PATENT-1 and their extension studies. Detailed descriptions of these studies can be found on the National Institutes of Health (NIH) website. At CHEST, exercise capacity was evaluated using the so-called 6-minute walk test.
Trade names
- Adempas (D, A, CH)
See also
- Cinaciguat , an sGC activator (not sGC stimulator).
- PDE-5 inhibitors reduce the degradation of cyclic GMP.
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Riociguat
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of methyl [4,6-diamino-2- [1- (2-fluorobenzyl) -1H-pyrazolo [3,4-b] pyridin-3-yl] pyrimidin-5- is shown, which is derived from a self-classification by the distributor yl] methyl carbamate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 11, 2020.
- ↑ Yoshina S, Tanaka A, Kuo SC: Studies on Heterocyclic Compounds. XXXVI. Synthesis of furo [3,2-c] pyrazole derivatives. (4) Synthesis of 1,3-diphenylfuro [3,2-c] pyrazole-5-carboxaldehyde and its derivatives (author's transl) . In: Yakugaku Zasshi . 98, No. 3, March 1978, pp. 272-9. PMID 650406 .
- ↑ Stasch JP, Becker EM, Alonso-Alija C, et al. : NO-independent regulatory site on soluble guanylate cyclase . In: Nature . 410, No. 6825, March 2001, pp. 212-5. doi : 10.1038 / 35065611 . PMID 11242081 .
- ↑ Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP: NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential . In: Nature Reviews Drug Discovery . 5, No. 9, September 2006, pp. 755-68. doi : 10.1038 / nrd2038 . PMID 16955067 . PMC 2225477 (free full text).
- ↑ Mittendorf J, Weigand S, Alonso-Alija C, et al. : Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension . In: ChemMedChem . 4, No. 5, May 2009, pp. 853-65. doi : 10.1002 / cmdc.200900014 . PMID 19263460 .
- ↑ Apotheke adhoc: Approval for Adempas . January 31, 2014. Archived from the original on July 17, 2014. Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved December 9, 2014.
- ↑ Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP: NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential . In: Nature Reviews. Drug Discovery . 5, No. 9, September 2006, pp. 755-68. doi : 10.1038 / nrd2038 . PMID 16955067 . PMC 2225477 (free full text).
- ↑ a b Grimminger F, Weimann G, Frey R, et al. : First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension . In: The European Respiratory Journal . 33, No. 4, April 2009, pp. 785-92. doi : 10.1183 / 09031936.00039808 . PMID 19129292 .
- ↑ Stasch JP, Hobbs AJ: NO-independent, haem-dependent soluble guanylate cyclase stimulators . In: Handbook of Experimental Pharmacology . 191, No. 191, 2009, pp. 277-308. doi : 10.1007 / 978-3-540-68964-5_13 . PMID 19089334 .
- ↑ Schermuly RT, Stasch JP, Pullamsetti SS, et al. : Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension . In: The European Respiratory Journal . 32, No. 4, October 2008, pp. 881-91. doi : 10.1183 / 09031936.00114407 . PMID 18550612 .
- ↑ a b Frey R, Mück W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G: Pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase activator cinaciguat (BAY 58-2667) in healthy male volunteers . In: Journal of Clinical Pharmacology . 48, No. 12, December 2008, pp. 1400-10. doi : 10.1177 / 0091270008322906 . PMID 18779378 .
- ↑ a b ClinicalTrials.gov: Riociguat .
- ↑ The 6-Minute Walk Test: An Inexpensive Alternative to Spiroergometry for Patients with Chronic Heart Failure? , in: Zeitschrift für Kardiologie , 2000, 89 (2), pp. 72-80, doi : 10.1007 / s003920050012 .
- ↑ ClinicalTrials.gov: A Study to Evaluate Efficacy and Safety of Oral BAY63-2521 in Patients With CTEPH .
