Robinson-Schöpf reaction
The Robinson-Schöpf reaction (also called Robinson-Schöpf condensation , Robinson synthesis or Robinson tropinone synthesis ) is a name reaction in organic chemistry . It is one of the multicomponent reactions and is used for the synthesis of tropinone . Their name goes back to the British chemist Robert Robinson (1886–1975) and the German chemist Clemens J. Schöpf (1899–1970) . The reaction was first described in the literature by Robinson in 1917 and improved by Schöpf in 1935. The German chemist succeeded in making the synthesis run under physiological conditions ( pH = 5–9) and thereby significantly increasing the yield.
Overview reaction
The reaction of methylamine , succinaldehyde and acetone dicarboxylic acid to form tropinone was originally called the Robinson-Schöpf reaction. This example shows the reaction:
It is also possible to use other aldehydes (apart from formaldehyde ) instead of succinaldehyde , whereby further tropane alkaloids can be obtained. This is discussed in more detail in the application examples section .
Reaction mechanism
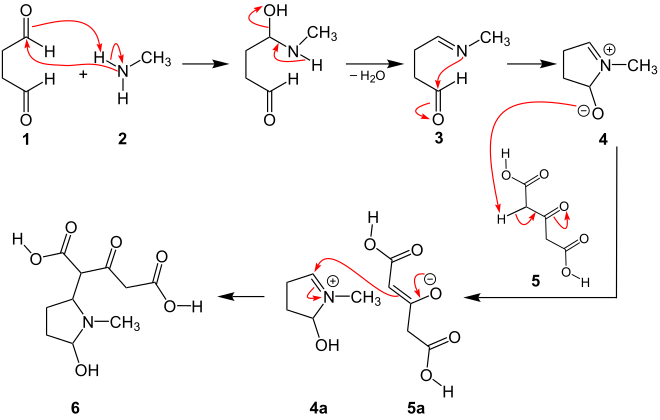
As already described above, a possible reaction mechanism will be presented using the example of the synthesis of tropinone.
From a formal point of view, the mechanism of the Robinson-Schöpf reaction can be described as a double Mannich reaction . In the first steps there is an intermolecular Mannich reaction. The succinaldehyde ( 1 ) is attacked nucleophilically by methylamine ( 2 ), with the formation of the imine with elimination of water ( 3 ). An intramolecular nucleophilic attack leads to the formation of the cyclic zwitterion ( 4 ) through ring closure . This cyclic zwitterion deprotonates the diacetone carboxylic acid ( 5 ), so that the enolate form of the acid is formed ( 5a ). In the next step, the enolate ( 5a ) is added to the cyclic iminium ion ( 4a ), whereby the first Mannich reaction with the intermediate ( 6 ) is completed.
Intermediate ( 6 ) can be graphed in several ways, all of which show the same molecule. The second part of the reaction mechanism is explained with variant ( iii ):
In the second part of the reaction mechanism, intermediate ( 6 ) leads directly to the second Mannich reaction, which takes place intramolecularly. A zwitterion ( 7 ) is also formed here with elimination of water . After formation of the enol form, a ring closure occurs again, so that the bicyclic basic structure ( 8 ) is created. The final product, tropinone ( 9 ), is formed by double β-decarboxylation :
.
Atomic economy
A chemical reaction has very good atom economy if only the desired main product is formed from the starting materials and no undesired by-products occur. So there is no waste. If one looks at the reaction mechanism of the Robinson-Schöpf reaction, it becomes clear that in the synthesis of tropinone two equivalents of water and two carbon dioxide are split off and are thus produced as waste. The atom economy can therefore be classified as rather mediocre, so that this name reaction does not run very efficiently from this point of view.
Application examples
The Robinson-Schöpf reaction is used to synthetically produce tropane alkaloids. In principle, it is possible to first produce tropinone using the classic Robinson-Schöpf reaction and, based thereon, for. B. to produce atropine . For this, first a reduction of the carbonyl group of the Tropinons to a hydroxy group . An alcohol is formed, which is then esterified with tropic acid.
In the classic Robinson-Schöpf reaction, succinaldehyde is used; if other aldehydes are used, further alkaloids can be produced. An example of this is the synthesis of pseudopelletierin from methylamine , glutaraldehyde and acetone dicarboxylic acid , which was developed by a group led by the American chemist Arthur C. Cope (1909–1966) :
Individual evidence
- ↑ a b c d Z. Wang: Comprehensive Organic Name Reactions and Reagents. Volume 3, John Wiley & Sons, Hoboken 2009, ISBN 978-0-471-70450-8 , pp. 2414-2417.
- ^ R. Robinson: LXIII. - A Synthesis of Tropinone. In: Journal of the Chemical Society . Volume 111, 1917, pp. 762-768, doi: 10.1039 / CT9171100762 .
- ↑ R. Robinson: LXXV.-A Theory of the Mechanism of the Phytochemical Synthesis of Certain alkaloid. In: Journal of the Chemical Society . Volume 111, 1917, pp. 876-899, doi: 10.1039 / ct9171100876 .
- ↑ a b C. Schöpf, G. Lehmann: The synthesis of tropinone, pseudopelletierins, lobelanins and related alkaloids under physiological conditions. In: Justus Liebig's Annals of Chemistry . Volume 518, No. 1, 1935, pp. 1-37, doi: 10.1002 / jlac.19355180102 .
- ↑ C. Schöpf: The synthesis of natural substances, especially alkaloids, under physiological conditions. and their significance for the question of the formation of some natural plant substances in the cell. In: Angewandte Chemie . Volume 50, No. 40, 1937, pp. 779-787, doi: 10.1002 / anie.19370504002 .
- ↑ LA Paquette , JH Home Aster: The stereo Chemical Course of a Robinson-Schöpf Biogenetic-type reaction. The Conformation of Certain Tricyclic Tropane Congeners. In: Journal of the American Chemical Society . Volume 88, No. 4, 1966, pp. 763-768, doi: 10.1021 / ja00956a029 .
- ^ BM Trost: On Inventing Reactions for Atom Economy. In: Accounts of Chemical Research . Volume 35, No. 9, 2001, pp. 695-705, doi: 10.1021 / ar010068z .
- ^ ROC Norman: Principles of Organic Synthesis. 2nd Edition. Chapman & Hall Ltd. , London 1978, p. 280.
- ^ AC Cope, HL Dryden, CF Howell: Pseudopelletierins. In: Organic Syntheses . Volume 37, 1957, p. 73, doi: 10.15227 / orgsyn.037.0073 .