Tropinone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
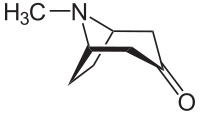
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Tropinone | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 13 NO | |||||||||||||||||||||
| Brief description |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 139.20 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.9872 g cm −3 (100 ° C ) |
|||||||||||||||||||||
| Melting point |
41-44 ° C |
|||||||||||||||||||||
| boiling point |
227 ° C |
|||||||||||||||||||||
| solubility |
bad in water |
|||||||||||||||||||||
| Refractive index |
1.4598 (100 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Tropinon is a synthetic alkaloid with a tropane core structure, which is also found in naturally occurring alkaloids such as. B. Cocaine and Atropine finds.
synthesis
The first synthesis of tropinone was carried out by Richard Willstätter in 1901. He used the apparently related cycloheptanone as a starting material, which he then used to produce tropinone in several steps, but only with a yield of 0.75%. Willstätter had previously been the first to synthesize cocaine from tropinone, which provided information about the chemical structure of cocaine.
The first total synthesis was carried out in 1917 by Robert Robinson and was improved in 1935 by Clemens Schöpf , which is why it is also known as the Robinson-Schöpf reaction . It is a multicomponent reaction . Tropinone is produced from the starting materials methylamine , succinaldehyde and acetone dicarboxylic acid .
Individual evidence
- ↑ a b c d e f g Tropinone data sheet (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-336.
- ^ W. Smit et al: Organic Synthesis, The Science behind the Art. The Royal Society of Chemistry, Cambridge 1998.
- ↑ Andrew J. Humphrey, David O'Hagan: Tropane alkaloid biosynthesis. A century old problem unresolved. In: Natural Products Reports. 18/2001, pp. 494-502.
- ^ Arthur J. Birch: Investigating a Scientific Legend: The Tropinone Synthesis of Sir Robert Robinson, FRS In: Notes and Records of the Royal Society of London. 47/1993, pp. 277-296.
- ↑ R. Robinson: A synthesis of tropinone. In: Journal of the Chemical Society . 111/1917, pp. 762-768.
- ↑ KC Nicolaou, Dionisios Vourloumis, Nicolas Winssinger, Phil S. Baran: The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century. In: Angew. Chem. Int. Ed. 39, 2000, pp. 44-122.
- ↑ C. Schöpf, G. Lehmann: The synthesis of tropinone, pseudopelletierins, lobelanins and related alkaloids under physiological conditions. In: Justus Liebig's Annals of Chemistry . Volume 518, No. 1, 1935, pp. 1-37, doi: 10.1002 / jlac.19355180102 .
- ^ Z. Wang: Comprehensive Organic Name Reactions and Reagents. Volume 3, John Wiley & Sons, Hoboken 2009, ISBN 978-0-471-70450-8 , pp. 2414-2417.

