Roush reaction
The Roush reaction (also called Roush crotylboration or Roush asymmetric allylation ) is a name reaction in organic chemistry . It goes back to the US chemist William R. Roush (* 1952) from 1985 and is used for the enantioselective production of chiral alcohols . The desired enantiomer can be obtained with an enantiomeric excess of 71-87%.
Overview reaction and stereoselectivity
The basic principle of the Roush reaction is the formation of an alcohol from an aldehyde with the formation of a new carbon-carbon bond. It is characteristic of this name reaction that the alcohol is a homoallyl alcohol. This is an alcohol in which the hydroxyl group is on the fourth carbon atom after a double bond. This homoallyl situation occurs because in the course of the reaction a new atom bond is made, which is marked in red in the picture . A rough overview reaction for the Roush reaction looks like this:
If this overview reaction is examined more closely, it becomes apparent that the aldehyde is a prochiral compound , whereas the homoallyl alcohol is a chiral compound in which the hydroxyl group is either syn or anti . Furthermore, it has not yet been conclusively clarified how the homoallyl position comes about. To clear up these ambiguities, the reaction needs to be considered more closely:
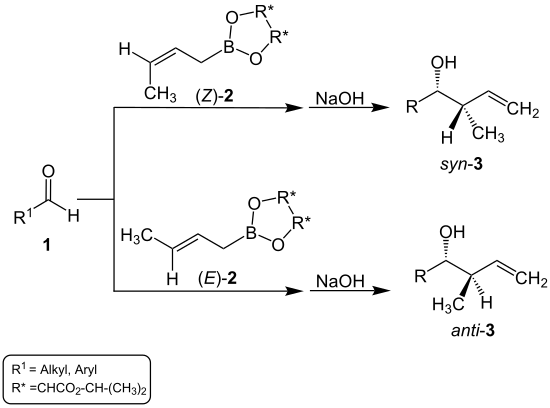
In addition to the aldehyde, Roush used a crotyl boronate for the synthesis of homoallyl alcohol:
This crotylboronate contains, on the one hand, chiral radicals which, together with substituents, e.g. B. a methyl group , on the third carbon atom in ( E ) or ( Z ) arrangement, determine the stereochemistry of the alcohol formed. On the other hand, this compound has an allyl group to which the aldehyde is added. This leads to the homoallyl position of the hydroxyl group in the alcohol which forms.
If a ( Z ) -crotylboronate [( Z ) - 2 ] is used, the main product is syn- homoallyl alcohol ( syn - 3 ). With an ( E ) -crotylboronate [( E ) - 2 ] an anti- homoallyl alcohol ( anti - 3 ) is formed as the main product. The overview reaction looks like this:
Assumptions about how the exact mechanism works and why the syn -homoallyl alcohol ( syn - 3 ) is formed as the main product in the reaction of ( Z ) - 2 and the anti- homoallyl alcohol ( anti - 3 ) as the main product with ( E ) - 2 , can be read at Roush and Kürti.
Atomic economy
From an atomic economic point of view, a chemical reaction can be regarded as efficient if all components of the starting materials are converted into the desired main product, as is usually the case with addition reactions . In the Roush reaction, however, the large boronate residue of the crotylboronate is produced as waste, so that this synthesis method is not necessarily recommended from the point of view of atom economy.
Application examples
The Roush reaction is widely used in the synthesis of asymmetric compounds. One example of this is the production of stevastelin B, a depsipeptide with immunosuppressive effects, which goes back to the Japanese Yukio Yamamoto and colleagues. The reaction presented in this article is used in conjunction with a similar reaction, the Evans-Aldol reaction, to synthesize stereocenters .
Individual evidence
- ^ A b c d e Z. Wang: Comprehensive Organic Name Reactions and Reagents. Volume 3, John Wiley & Sons, Hoboken 2009, ISBN 978-0-471-70450-8 , pp. 2435-2438.
- ↑ a b c d W. R. Roush, AE Walts, LK Hoong: Diastereo- and enantioselective aldehyde addition reactions of 2-allyl-1,3,2-dioxaborolane-4,5-dicarboxylic esters, a useful class of tartrate ester modified allylboronates. In: Journal of the American Chemical Society . Volume 107, No. 26, 1985, pp. 8186-8190, doi: 10.1021 / ja00312a062 .
- ↑ a b c d e f L. Kürti, B. Czakó: Strategic Applications of Named Reactions in Organic Synthesis. Elsevier Academic Press, Burlington / San Diego / London 2005, ISBN 0-12-369483-3 , pp. 386-387.
- ^ S. Kubik: Entry on Homoallyl position. In: Römpp Online . Georg Thieme Verlag, accessed on August 1, 2017.
- ^ BM Trost: The atom economy - a search for synthetic efficiency. In: Science . Volume 254, No. 5037, 1991, pp. 1471-1477, doi: 10.1126 / science.1962206 .
- ↑ N. Kohyama, Y. Yamamoto: Total Synthesis of Stevastelin B, a Novel Immunosuppressant. In: Synlett . Volume 2001, No. 5, 2001, pp. 694-696, doi: 10.1055 / s-2001-13382 .