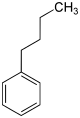
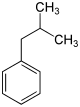
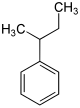
In chemistry, the butylbenzenes form a group of four aromatic hydrocarbons with all four variants of the butyl group as substituents on the benzene . Their different arrangement results in four constitutional isomers with the empirical formula C 10 H 14 . They also belong to the group of C 4 benzenes .
presentation
Starting from benzene achieved under catalysis with anhydrous aluminum chloride , the representation of tert -butylbenzene with 2-chloro-2-methylpropane ( tert -butyl chloride) as the electrophile in a Friedel-Crafts alkylation :
Web links
Individual evidence
-
↑ a b c d e f g h i j k l m Entry on n-butylbenzene in the GESTIS substance database of the IFA , accessed on March 14, 2018(JavaScript required) .
-
↑ a b butylbenzene data sheet from Sigma-Aldrich , accessed on November 27, 2012 ( PDF ).
-
↑ Isobutylbenzene data sheet from Sigma-Aldrich , accessed on November 27, 2012 ( PDF ).
-
↑ a b c d e f g h i j k l m n Entry on isobutylbenzene in the GESTIS substance database of the IFA , accessed on March 14, 2018(JavaScript required) .
-
↑ a b c d e f g h i j k l m n o Entry on sec-butylbenzene in the GESTIS substance database of the IFA , accessed on March 14, 2018(JavaScript required) .
-
↑ a b c d e f g h i j k l m n Entry on tert-butylbenzene in the GESTIS substance database of the IFA , accessed on March 14, 2018(JavaScript required) .
-
↑ a b data sheet tert-butylbenzene from Sigma-Aldrich , accessed on November 27, 2012 ( PDF ).
-
↑ CRC Handbook of Tables for Organic Compound Identification . 3. Edition. 1984, ISBN 0-8493-0303-6 .