Selectfluor
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
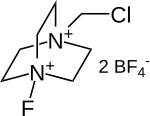
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Selectfluor | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 14 B 2 ClF 9 N 2 | |||||||||||||||
| Brief description |
White dust |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 354.26 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
260 ° C |
|||||||||||||||
| solubility |
Easily soluble in water (176 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Selectfluor is a fluorinating reagent and a derivative of DABCO . Selectfluor was first described by Eric Banks and co-workers in 1992 and has been used for electrophilic fluorination ever since. The compound is extremely stable and less toxic than pure fluorine .
Extraction and presentation
The synthesis of Selectfluor begins with the alkylation of DABCO with dichloromethane . The chloride counterions are then exchanged with sodium tetrafluoroborate . The sodium chloride is precipitated from the acetonitrile solution . The intermediate product is fluorinated with F 2 in the last step .

use
Selectfluor has a wide range of uses in organofluorochemistry as a source of electrophilic fluorine as well as in radical fluorination .
Individual evidence
- ↑ a b Data sheet Selectfluor ® fluorinating reagent,> 95% in F + active from Sigma-Aldrich , accessed on January 20, 2015 ( PDF ).
- ↑ a b c Entry on 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo (2.2.2) octane-bis-tetrafluoroborate) in the GESTIS substance database of the IFA , accessed on February 5, 2017(JavaScript required) .
- ↑ Entry on 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo [2.2.2] octanbis (tetrafluoroborate) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 13, 2017. Manufacturers or distributors can use the expand harmonized classification and labeling .
- ↑ a b c d Paul T. Nyffeler, Sergio Gonzalez Durón, Michael D. Burkart, Stéphane P. Vincent, Chi-Huey Wong: Selectfluor: Mechanisms and Applications . In: Angewandte Chemie . tape 117 , no. 2 , January 2005, p. 196-217 , doi : 10.1002 / anie.200400648 .
- ^ R. Eric Banks, Suad N. Mohialdin-Khaffaf, G. Sankar Lal, Iqbal Sharif, Robert G. Syvret: 1-Alkyl-4-fluoro-1,4-diazoniabicyclo [2.2.2] octane salts: a novel family of electrophilic fluorinating agents . In: Journal of the Chemical Society, Chemical Communications . No. 8 , 1992, pp. 595 , doi : 10.1039 / C39920000595 .
- ↑ Laxmi Manral: Selectfluor (F-TEDA-BF 4 ) C 7 H 14 B 2 ClF 9 N 2 . In: Synlett . No. 5 , 2006, p. 0807-0808 , doi : 10.1055 / s-2006-933124 .



