Seyferth-Gilbert chain extension
The Seyferth-Gilbert chain extension , also known as the Seyferth-Gilbert reaction or Seyferth-Gilbert homologation , is a name reaction in organic chemistry . It was named after Dietmar Seyferth , who published it in 1970. In 1979 the reaction of John C. Gilbert et al. examined more closely. In this reaction, aldehydes or ketones react to form internal or terminal alkynes .
Overview
In the presence of potassium tert -butanolate and dimethyl (diazomethyl) phosphonate (also known as Seyferth-Gilbert reagent ), aldehydes and ketones react to form alkynes. This reaction is also called homologation or chain extension because after the reaction there is exactly one more carbon atom (marked in blue in the overview ) between the residues R 1 and R 2 than before.

If an aldehyde is used as the starting material, a terminal alkyne (1-alkyne) is obtained. If the starting material is a ketone (i.e. R 1 ≠ H ≠ R 2 ) the C≡C triple bond is further inside the molecule.
mechanism
The tert- butanolate acts as a base and deprotonates the Seyferth-Gilbert reagent 1 , so that a carbanion 2 is formed. The negatively charged carbon atom 2 now attacks the carbonyl group of the aldehyde or the ketone, which leads to the formation of an alcoholate 3 . This is followed by an intramolecular attack by the negatively charged oxygen 3 on the phosphorus atom . This creates the four-ring intermediate 4 under ring closure . With elimination of a dimethyl phosphate, an intermediate is formed in the form of a diazovinyl compound 5 . Nitrogen is split off from this intermediate product and a carbene 6 is formed . This is followed by a rearrangement of the radical R 2 and thus the formation of an alkyne 7 as a product.
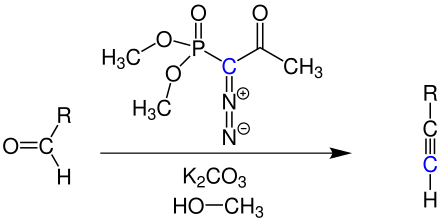
Bestmann modification
The Seyferth-Gilbert reaction was modified by Susumu Ohira and Hans Jürgen Bestmann . The generation of dimethyl (diazomethyl) phosphonate in situ by the reaction of dimethyl (1-diazo-2-oxopropyl) phosphonate (also known as Ohira-Bestmann reagent ) with methanol and potassium carbonate ensures a high yield in the synthesis of terminal Alkynes. Here, too, R corresponds to an organic residue.
One advantage of the Bestmann modification is that the reaction conditions are very mild and therefore many functional groups are tolerated.
See also
Individual evidence
- ↑ a b c d Zerong Wang: Comprehensive organic name reactions and reagents . John Wiley & Sons, Hoboken, NJ 2009, ISBN 978-0-471-70450-8 , pp. 2559-2560 .
- ↑ D. Seyferth, RS Marmor: Dimethyl diazomethylphosphonate: its preparation and reactions . In: Tetrahedron Letters . tape 11 , no. 28 , 1970, pp. 2493-2496 , doi : 10.1016 / S0040-4039 (01) 98264-4 .
- ↑ D. Seyferth, RS Marmor, P. Hilbert: Reactions of dimethylphosphono-substituted diazoalkanes. (MeO) 2 P (O) CR transfer to olefin and 1,3-dipolar additions of (MeO) 2 P (O) C (N2) R . In: J. Org. Chem. Band 36 , no. 10 , 1971, p. 1379-1386 , doi : 10.1021 / jo00809a014 .
- ↑ JC Gilbert, U. Weerasooriya: Elaboration of aldehydes and ketones to alkynes: improved methodology . In: J. Org. Chem. Band 44 , no. 26 , 1979, pp. 4997-4998 , doi : 10.1021 / jo00394a063 .
- ↑ JC Gilbert, U. Weerasooriya: Diazoethenes: their attempted synthesis from aldehydes and aromatic ketones by way of the Horner-Emmons modification of the Wittig reaction. A facile synthesis of alkynes . In: J. Org. Chem. Band 47 , no. 10 , 1982, pp. 1837-1845 , doi : 10.1021 / jo00349a007 .
- ↑ a b c d e László Kürti, Barbara Czakó: Strategic applications of named reactions in organic synthesis: background and detailed mechanisms . Elsevier Academic Press, Amsterdam / Boston 2005, ISBN 0-12-429785-4 , pp. 402-403 .
- ^ S. Müller, B. Liepold, GJ Roth, HJ Bestmann: An Improved One-pot Procedure for the Synthesis of Alkynes from Aldehydes . In: Synlett . tape 6 , 1996, pp. 521-522 , doi : 10.1055 / s-1996-5474 .
- ↑ Methanolysis of Dimethyl (1-Diazo-2-oxopropyl) Phosphonate: Generation of Dimethyl (Diazomethyl) Phosphonate and Reaction with Carbonyl Compounds . In: Synthetic Communications: An International Journal for Rapid Communication of Synthetic Organic Chemistry . tape 19 , no. 3-4 , 1989, pp. 561-564 , doi : 10.1080 / 00397918908050700 .
- ^ GJ Roth, B. Liepold, SG Müller, HJ Bestmann: Further Improvements of the Synthesis of Alkynes from Aldehydes . In: Synthesis . tape 1 , 2004, p. 59-62 , doi : 10.1055 / s-2003-44346 .