Sinistrin
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
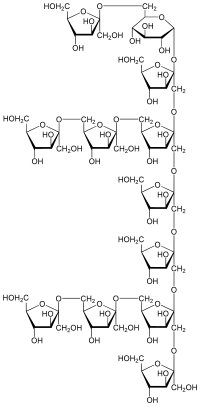
| One possible structure of Sinistrin | |||||||||
| General | |||||||||
| Surname | Sinistrin | ||||||||
| other names |
|
||||||||
| CAS number | 37311-25-4 | ||||||||
| Monomers / partial structures | Fructose | ||||||||
| ATC code | |||||||||
| Brief description |
slightly yellow colored, flour-like powder |
||||||||
| properties | |||||||||
| Physical state |
firmly |
||||||||
| Melting point |
Decomposes at 160-170 ° C |
||||||||
| solubility |
|
||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Sinistrin (also known as polyfructosan ) is a naturally occurring polysaccharide in many plants . Like the similar fructan inulin, Sinistrin serves as an energy store ( storage carbohydrate ) in plants.
History of discovery and manufacture
In 1879 Schmiedeberg succeeded in isolating a new carbohydrate from the tuber of the red sea onion ( Urginea maritima ). He gave the left-turning substance the name "Sinistrin", after the Latin word sinister for "left". Hammarsten found the Sinistrin in 1885 in the mucins of the Roman snail ( Helix pomatia ). Today Sinistrin is primarily obtained industrially from the red sea onion through several extraction and purification steps.
Chemical structure and properties
Sinistrin is a β– D- fructan of the inulin type with branches at position 6. Like inulin, it counts to the fructans or is partly also to the fructooligosaccharides ( FOS ). Sinistrin consists of approx. 97% fructose and approx. 3% glucose , the chain being composed of fructose molecules that have a terminal glucose residue. The degree of polymerisation of Sinistrin is on average 15, the molar mass 3500 Da , with a range between 2000 and 6000 Da.
Sinistrin differs from inulin by its high solubility in water (even in cold water) and better alkali stability. When an aqueous sinistrin solution is mixed with copper sulphate solution and then with potassium hydroxide solution , a deep blue colored solution is created. If this is heated, a light blue, flaky precipitate is immediately deposited; the reaction can be used as sensitive detection for sinistrin. Sinistrin solution forms a precipitate with calcium hydroxide or milk of lime .
Use in medicine
Like inulin, sinistrin is used in physiological research to determine extracellular space, as it easily penetrates the interstitium , but not the cells. Sinistrin is completely filtered in the glomerulum , but is neither secreted nor reabsorbed in the tubule system. It is therefore excreted unchanged and completely through the kidneys. The measurement of the sinistrin clearance is therefore used for the exact determination of the glomerular filtration rate ( GFR ) of the kidneys. This excretion rate provides information about the activity and state of health of the kidneys. The quantitative determination of sinistrin in urine and plasma is identical to that of inulin. For this area of application, Sinistrin is approved as an aqueous solution under the trade name "Inutest" as a medicinal product.
literature
- DP Mertz, H. Sarre: Polyfructosan-S: A new inulin-like substance for the determination of the glomerular filtrate and the physiologically active extracellular fluid volume in humans . Klin Wochenschrift, 1963, 41: 868-872.
Individual evidence
- ↑ a b c d e M. Geldmacher-Mallinckrodt and F. May: The polysaccharides of the Roman snail II. The mucin polysaccharides of the protein gland. 1st communication: the identity of galactogen and sinistrin. Hoppe-Seyler's journal for physiological chemistry. Volume 307, Issue 1–2, pp. 191–201, doi : 10.1515 / bchm2.1957.307.1-2.191 .
- ↑ a b c O. Schmiedeberg: About a new carbohydrate. In: Hoppe-Seyler's magazine for physiological chemistry , Volume 3 (1-2), 1879. doi : 10.1515 / bchm1.1879.3.1-2.112 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Biopolymers, Polysaccharides II , Wiley-VCH, 2002, ISBN 3-527-30227-1 .
- ↑ E. Nitsch, W. Ivanov and K. Lederer: Molecular characterization of Sinistrin . Carbohydrate Research 72 (1979), 1-12.
- ↑ T. Spies, W. Praznik u. a .: The structure of the fructan sinistrin from Urginea maritima. In: Carbohydrate research. Volume 235, November 1992, pp. 221-230, PMID 1473105 .
- ↑ B. Watschinger and I. Kobinger: Wiener Journal of Medicine 45 (1964), 219-228.
- ↑ T. Buclin, A. Pechere-Bertschi, R. Sechaud et al .: Sinistrin clearance for determination of glomerular filtration rate: a reappraisal of various approaches using a new analytical method . In: J Clin Pharmacol . 1997; 37: 679-92. 204-211.
- ↑ Inutest 25% ampoules (PDF; 60 kB) at pharmazie.com, accessed on July 7, 2011.