Tantalum (IV) iodide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| __ Ta 4+ __ I - | |||||||
| General | |||||||
| Surname | Tantalum (IV) iodide | ||||||
| other names |
Tantalum triiodide |
||||||
| Ratio formula | TaI 4 | ||||||
| Brief description |
black solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 688.57 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
398 ° C |
||||||
| solubility |
reacts with water |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Tantalum (IV) iodide is an inorganic chemical compound of tantalum from the group of iodides .
Extraction and presentation
Tantalum (IV) iodide can be obtained by reacting tantalum (V) iodide with aluminum , magnesium or calcium at 380 ° C. This also creates Ta 6 I 14 as a stable soil body . The preparation of a very pure crystallized tantalum (IV) iodide is therefore difficult.
properties
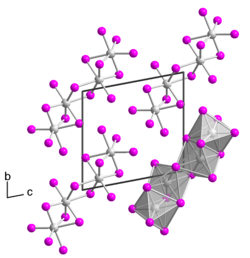
Tantalum (IV) iodide is a black solid. It has a crystal structure isotypic to that of niobium (IV) iodide . Monocrystalline tantalum (IV) iodide was first obtained in 2008 from Rafal Wiglusz and Gerd Meyer as a chance product of a conversion in a tantalum ampoule, which should actually lead to the product Rb (Pr 6 C 2 ) I 12 . The single crystal has a triclinic crystal structure with the space group P 1 (space group no.2 ) with two formula units per unit cell (a = 707.36 pm, b = 1064.64 pm, c = 1074.99 pm, α = 100.440 °, β = 89.824 ° and γ = 104.392 °). The crystal structure is different from that of other Übergangsmetalltetraiodide who usually a MI 2.4 I 2.1 have -Kettenstruktur as they made TaI 6 - octahedra is bridged by a common surface to form a dimer. Two such dimers bridge a common edge to form a tetramer .
Individual evidence
- ↑ a b c d e Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1455 .
- ↑ WM Haynes (Ed.): CRC handbook of chemistry and physics. A ready-reference book of chemical and physical data . founded by David R. Lide. 93rd edition. CRC Press, Boca Raton 2012, ISBN 978-1-4398-8049-4 , pp. 4–93 (English, limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Gerd Meyer, Rafal Wiglusz, Ingo Pantenburg, Anja-Verena Mudring: Tantalum (IV) Iodide, TaI4: A Molecular Solid Consisting of Dimers of Dimers, Ta4I16. In: Journal of Inorganic and General Chemistry. 634, 2008, pp. 825-828, doi : 10.1002 / zaac.200700529 .
- ↑ Katja Habermehl; New studies on halides of niobium and tantalum (PDF; 3.9 MB), urn : nbn: de: hbz: 38-31032 , April 22, 2010.
