Tetrabutyl germanium
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
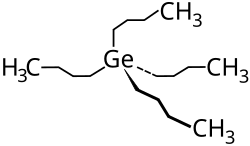
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrabutyl germanium | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 16 H 36 Ge | |||||||||||||||
| Brief description |
colorless |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 301.06 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.930 g cm −3 |
|||||||||||||||
| boiling point |
100 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrabutylgermanium is a chemical compound from the group of organic germanium compounds .
Extraction and presentation
Organic germanium compounds can be prepared analogously to the corresponding silicon compounds. Correspondingly, tetrabutylgermanium can be produced by reacting germanium tetrachloride with butyllithium :
properties
Physical Properties
Tetrabutylgermanium has a flash point of 113 ° C and a refractive index of 1.455. The 73 Ge NMR shift is 25.4 ppm .
Chemical properties
With a catalytic conproportionation of tetrabutylgermanium with germanium tetrachloride in the presence of aluminum chloride as a catalyst , tributylgermanium chloride and dibutylgermanium dichloride are formed depending on the ratio :
When heated, tetrabutylgermanium reacts with elemental sulfur at 230 ° C to form a cyclic trithiator man in which the six-membered ring consists of alternating sulfur and germanium atoms and two butyl groups are bonded to the germanium atoms:
Individual evidence
- ↑ a b c d e f g data sheet Tetrabutylgermanium from Sigma-Aldrich , accessed on April 12, 2015 ( PDF ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 958.
- ^ Entry on TETRA-N-BUTYLGERMANE at ChemicalBook , accessed on April 12, 2015.
- ↑ J. Kaufmann, W. Sahm, A. Schwenk: 73 Ge Nuclear Magnetic Resonance Studies. In: Journal of Nature Research A . 26, 1971, pp. 1384-1389 ( PDF , free full text).
- ↑ GJM van der Kerk, F. Rijkens, MJ Janssen: Investigations on organogermanium compounds I: On the redistribution reaction between tetra-n-butylgermanium and germanium tetrachloride . In: Recueil des Travaux Chimiques des Pays-Bas . tape 81 , no. 9 , 1962, ISSN 0165-0513 , pp. 764 , doi : 10.1002 / recl.19620810907 .
- ^ Max Schmidt, Herbert Schumann: Cleavage reactions of organometallic compounds with chalcogens. Reactions of sulfur with organosilicon, germanium and lead organic compounds . In: Journal of Inorganic and General Chemistry . tape 325 , no. 3-4 , October 1963, ISSN 0044-2313 , pp. 130 , doi : 10.1002 / zaac.19633250305 .


![{\ mathrm {3 \ Ge (C_ {4} H_ {9}) _ {4} + \ GeCl_ {4} \ {\ xrightarrow [{}] {AlCl_ {3}}} \ 4 \ ClGe (C_ {4 } H_ {9}) _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e002161b873c6de9ee6138561f6198c7892a4d64)
![{\ mathrm {Ge (C_ {4} H_ {9}) _ {4} + \ GeCl_ {4} \ {\ xrightarrow [{}] {AlCl_ {3}}} \ 2 \ Cl_ {2} Ge (C_ {4} H_ {9}) _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1df37a6046f0294b143ea21329b716031c3b0322)
![{\ mathrm {3 \ Ge (C_ {4} H_ {9}) _ {4} + \ 6 \ S \ {\ xrightarrow [{}] {}} \ 3 \ S ((C_ {4} H_ {9 }) _ {3}) _ {2} + \ (SGe (C_ {4} H_ {9}) _ {2}) _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9d6534e5f40a022370c9616a3b543003bb4f44e5)