Germanium (IV) chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
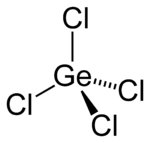
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Germanium (IV) chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | GeCl 4 | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 214.45 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.88 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
−49.5 ° C |
|||||||||||||||
| boiling point |
83 ° C |
|||||||||||||||
| Vapor pressure |
101 h Pa (21 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−531 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Germanium (IV) chloride is a chemical compound from the group of germanium compounds and chlorides .
Extraction and presentation
Germanium (IV) chloride can be obtained by reacting germanium or germanium (IV) oxide / germanium (II) oxide with chlorine or hydrogen chloride .
properties
Germanium (IV) chloride is a colorless, air-smoking liquid with a pungent odor (from hydrochloric acid). It has a boiling point of 83 ° C and slowly hydrolyzes with water or acids to form germanium (IV) oxide. The corresponding germanium diimide is obtained by aminolysis . The extremely hydrolysis-sensitive orthoethyl germanic acid is formed with sodium ethanolate .
With chlorides it forms chloro complexes of the type GeCl 6 2− .
use
Germanium (IV) chloride is an important intermediate in germanium extraction and the microwave production of Hexachlordigerman Ge 2 Cl 6 . Highly pure germanium (IV) chloride is used in the production of optical waveguides made of quartz glass in order to create a high-purity germanium (IV) oxide layer in the core of the quartz fibers .
Individual evidence
- ↑ Germanium (IV) chloride data sheet (PDF) from Merck , accessed on June 14, 2011.
- ↑ a b c d e f g h data sheet Germanium (IV) chloride at Sigma-Aldrich , accessed on July 29, 2017 ( PDF ).
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 721.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 1171 (reading sample: Part A - Basics of the chemistry of hydrogen. Google book search ).
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag (2011) pp. 68–69, ISBN 978-3-8348-1245-2 .
- ^ Robert Schwarz: Contributions to the chemistry of germanium. (I. Communication) . In: Reports of the German Chemical Society (A and B Series) . tape 62 , no. 9 , October 9, 1929, p. 2477 , doi : 10.1002 / cber.19290620902 .
- ^ Robert Schwarz, Peter W. Schenk: Contributions to the chemistry of Germanium, 2nd part: Germanium-nitrogen compounds . In: Reports of the German Chemical Society (A and B Series) . tape 63 , no. 2 , February 5, 1930, p. 296 , doi : 10.1002 / cber.19300630204 .
- ^ Robert Schwarz, PW Schenk, H. Giese: Contributions to the chemistry of Germanium (VI.) . In: Reports of the German Chemical Society (A and B Series) . tape 64 , no. 2 , February 4, 1931, p. 362 , doi : 10.1002 / cber.19310640227 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1015.
- ^ Germanium tetrachloride (RMO GmbH) ( Memento from February 12, 2013 in the web archive archive.today ).
Web links
- RL Benoit, J. Place: Fluoride complexes of germanium (IV) in aqueous solution . In: Canadian Journal of Chemistry . 41 (5), 1963, pp. 1170-1180, doi : 10.1139 / v63-165 .






