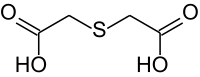
Thiodiglycolic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Thiodiglycolic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 6 O 4 S | |||||||||||||||
| Brief description |
beige powder with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 150.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
128-131 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Thiodiglycolic acid is an organic compound. It consists of two acetic acid groups that are linked by a sulfur bridge.
Extraction
Thiodiglycolic acid is obtained from hydrogen sulfide and sodium chloroacetate :
properties
Thiodiglycolic acid is a flammable beige solid with an unpleasant odor that is easily soluble in water. Your aqueous solution reacts strongly acidic.
use
Thiodiglycolic acid is used as a detection reagent for copper, lead, mercury and silver.
The salts of thiodiglycolic acid are called thiodiglycolates and are used in many ways. Dibutyl thioglycolate is a raw material for the synthesis of polythiophenes (electrically conductive polymers). Di (2-ethylhexyl) thiodiglycolate is used as a plasticizer in rubber production ( Vulkanol 90 ).
Medical importance
In the event of poisoning with vinyl chloride or treatment with ifosfamide , thiodiglycolic acid can be detected and quantified in the urine.
Individual evidence
- ↑ a b c d e f g Entry on thiodi (acetic acid) in the GESTIS substance database of the IFA , accessed on March 27, 2013(JavaScript required) .
- ↑ a b c d Entry on thiodiglycolic acid in the Hazardous Substances Data Bank , accessed on March 27, 2013.
- ↑ Data sheet 2,2′-thiodiacetic acid (PDF) from Merck , accessed on March 27, 2013.
- ↑ bruno bock: thiodiglycolic , accessed on September 4 2016th
- ↑ Theresa M. Visarius, Heinz Bähler, Adrian Küpfer, Thomas Cerny, H. Bernhard Lauterburg: Thiodiglycolic acid is excreted by humans receiving ifosfamide and Inhibits mitochondrial function in rats . In: Drug Metabolism and Disposition . tape 26 , no. 3 , 1998, p. 193-196 , PMID 9492379 ( PDF ).

