Tiglic aldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
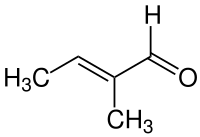
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tiglic aldehyde | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 8 O | |||||||||||||||
| Brief description |
colorless to yellowish odorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 84.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.87 g cm −3 |
|||||||||||||||
| boiling point |
116 ° C |
|||||||||||||||
| Vapor pressure |
95 hPa (50 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.448 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Tigline aldehyde is a chemical compound from the group of aldehydes with an additional double bond .
Occurrence
Tiglic aldehyde occurs naturally in guaiac resin , geranium oil, and other natural substances.
Extraction and presentation
The compound can be obtained by reacting acetaldehyde and propionaldehyde . In addition, it isomerizes from angelica aldehyde .
properties
Tigline aldehyde is a highly flammable, colorless to yellowish odorless liquid that is practically insoluble in water.
use
Tigline aldehyde is used as an intermediate in the manufacture of other chemical compounds.
safety instructions
The vapors of tiglinic aldehyde can form an explosive mixture with air ( flash point approx. 13 ° C).
Individual evidence
- ↑ a b c d e f g h i j Entry on tiglic aldehyde in the GESTIS substance database of the IFA , accessed on April 5, 2017(JavaScript required) .
- ↑ a b George A. Burdock: Fenaroli's Handbook of Flavor Ingredients, Fifth Edition . CRC Press, 2004, ISBN 978-1-4200-3787-6 , pp. 1147 ( limited preview in Google Book search).
- ↑ Data sheet Tiglic aldehyde, ≥96% from Sigma-Aldrich , accessed on April 5, 2017 ( PDF ).
- ↑ Fred Winter: Handbook of the entire perfumery and cosmetics A scientific-practical presentation of the modern perfumery including the production of the toilet soaps as well as an outline of the applied cosmetics . Springer-Verlag, 2013, ISBN 978-3-662-38143-4 , p. 945 ( limited preview in Google Book search).
- ↑ Hans Meyer: Synthesis of the carbon compounds . Springer-Verlag, 2013, ISBN 978-3-662-36309-6 , pp. 73 ( limited preview in Google Book search).
- ↑ Dictionary of Food Compounds , p. 1277 ( limited preview in Google book search).
- ↑ Data sheet trans-2-methyl-2-butenal, 97% from AlfaAesar, accessed on April 5, 2017 ( PDF )(JavaScript required) .

