Traubean synthesis
The grape-ash-purine synthesis , or grape synthesis , is a versatile process for the synthesis of purine and its derivatives from pyrimidine derivatives . It is a name reaction of organic chemistry named after the German chemist Wilhelm Traube (1866–1942) . It was published for the first time in 1900.
Nowadays it is mainly used industrially to produce caffeine in several steps from cyanoacetic acid and urea .
mechanism
The mechanism is demonstrated here using the reaction of 5,6-diaminopyrimidin-4-ol and formic acid as an example :
First of all, formic acid is added to the pyrimidine derivative 1 . This attaches to the pyrimidine derivative, so that the unstable diol 2 is obtained. The amide 3 is formed from this by splitting off water . A cyclization now takes place through an intramolecular , nucleophilic attack , with the reactive intermediate 4 being formed. The diol 5 is formed by intramolecular [1,3] proton transfer . This reacts by splitting off water to form the purine derivative 6 .
It is extremely important that a very pure pyrimidine is always used as the starting material, since otherwise undesirable by-products can be formed. But even under these conditions it can happen that no purine derivatives are obtained. This is due to the fact that the starting material only reacts up to the formylation , but does not cyclize afterwards.

Caffeine production
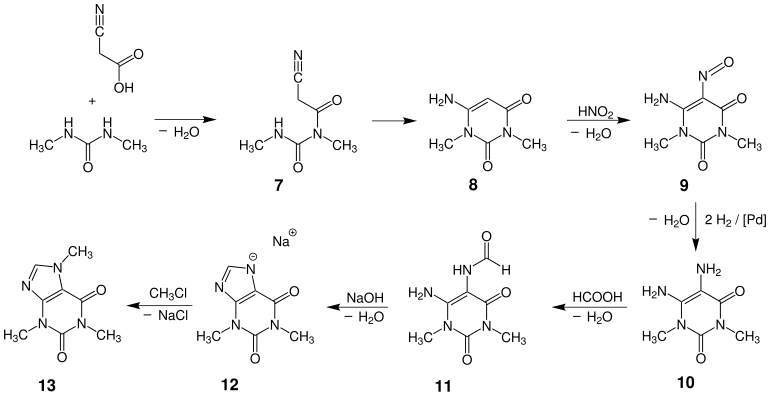
The industrial synthesis of caffeine starting from cyanoacetic acid and N , N ′ -dimethylurea is one of the most important applications of the grape-purine synthesis .
Synthesis of caffeine
Starting from cyanoacetic acid and dimethylurea, the synthesis begins with a condensation of the amide nitrogen on the carboxy group of the cyanoacetic acid, which leads to the formation of amide 7 . The cyclization proceeds through a nucleophilic attack of the second nitrogen on the nitrile , whereby the compound 8 , a derivative of uracil , is obtained. Subsequent nitrosylation with nitrous acid converts this to the nitroso compound 9 , which is reduced to the amine 10 in the next step by hydrogen on a platinum catalyst . Subsequent formylation of an amino group with formic acid gives formamide 11 , which is cyclized in the next step by the action of sodium hydroxide solution to form the imidazolium salt 12 , the sodium salt of theophylline . Starting from this salt , caffeine 13 can be obtained by reaction with chloromethane .
There are many other uses for this reaction. So z. B. a modification with the help of which the condensation of a 4,5-diaminopyrimidine with an aldehyde could be improved to a one-step process in which only weak bases have to be used and an accompanying oxidation with air takes place.
literature
- JJ McKetta, WA Cunningham: Encyclopedia of Chemical Processing and Design. Volume 5: Blowers to Calcination. 1st edition, Marcel Dekker Inc., 1977, ISBN 0824724550 , pp. 435-437.
Individual evidence
- ↑ W. Traube: The synthetic structure of uric acid, xanthine, theobromine, theophylline and caffeïns from cyanoacetic acid. in: Chem. Ber. 33, 1900, pp. 3035-3056; doi: 10.1002 / cber.19000330352 .
- ^ A b c Z. Wang: Comprehensive Organic Name Reactions and Reagents. 3 volumes. John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 2789-2792.
- ^ R. Robins, K. Dille, C. Willits, B. Christensen: Purines. II. The Synthesis of Certain Purines and the Cyclization of Several Substituted 4,5-Diaminopyridines - Correction . In: Journal of the American Chemical Society . tape 75 , no. 24 , 1953, pp. 6359-6359 , doi : 10.1021 / ja01120a618 .
- ↑ Aleem Gangjee, Anil Vasudevan, and Sherry F. Queener: Conformationally Restricted Analogues of Trimethoprim: 2,6-Diamino-8-substituted Purines as Potential Dihydrofolate Reductase Inhibitors from Pneumocystis carinii and Toxoplasma gondii1 . In: Journal of Medicinal Chemistry . tape 40 , no. 19 , 1997, pp. 3032-3039 , doi : 10.1021 / jm970271t .