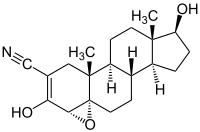
Trilostane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Trilostane | ||||||||||||||||||
| other names |
(2 α , 4 α , 5 α , 17 β ) -4,5-epoxy-17-hydroxy-3-oxoandrostane-2-carbonitrile |
||||||||||||||||||
| Molecular formula | C 20 H 27 NO 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class |
Anticorticosteroid |
||||||||||||||||||
| Mechanism of action |
Inhibition of steroid hormone synthesis |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 329.43 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Trilostane is the generic name of a chemical compound belonging to the steroid class that is used in veterinary medicine to treat hypophyseal hypophysis . Trilostane inhibits the 3 β -hydroxysteroid dehydrogenase enzyme system and thus competitively the synthesis of various steroid hormones (e.g. cortisol and aldosterone ) in the adrenal cortex , but is itself hormonally inactive. The drug is well tolerated and works very safely, quickly and reversibly. The side effects are significantly fewer than with mitotane .
The biotransformation takes place in the liver . The plasma half-life is eight hours.
It is used to treat Cushing's Syndrome in dogs and horses ( Equines Cushing's Syndrome , ECS) and Alopecia X in some breeds of dogs.
Trade names
Modrenal, Vetoryl
Web links
- Entry on Trilostane at Vetpharm
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of trilostane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on September 24, 2018, is reproduced from a self-classification by the distributor .