Trimethylsilyl azide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
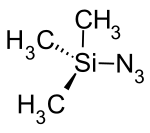
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Trimethylsilyl azide | ||||||||||||||||||
| other names |
Azidotrimethylsilane |
||||||||||||||||||
| Molecular formula | C 3 H 9 N 3 Si | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 115.21 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.868 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
−95 ° C |
||||||||||||||||||
| boiling point |
52-53 ° C (175 mmHg), 95 ° C (720 mmHg) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.4142 (20 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Trimethylsilyl azide is a chemical compound from the group of azides .
Extraction and presentation
Trimethylsilyl azide can be obtained by reacting chlorotrimethylsilane with sodium azide in quinoline .
properties
Trimethylsilyl azide is a colorless, thermally stable, but hydrolysis-sensitive liquid.
use
Trimethylsilyl azide can be used to prepare carboxylic acid azides from carboxylic acid chlorides and alkynyl isocyanate compounds. Beryllium azide can be obtained by reacting with beryllium chloride .
Individual evidence
- ↑ a b c d e f Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 710.
- ↑ a b c e-EROS Encyclopedia of Reagents for Organic Synthesis , 1999-2013, John Wiley and Sons, Inc., entry for Azidotrimethylsilane, accessed May 26, 2020 .
- ↑ a b c data sheet trimethylsilylazide, 95% from Sigma-Aldrich , accessed on March 20, 2013 ( PDF ).
- ↑ a b Data sheet trimethylsilylazide (PDF) from Merck , accessed on March 20, 2013.
- ↑ Eberhard Breitmaier, Günther Jung: Organic chemistry . Georg Thieme Verlag, 2005, ISBN 3-13-541505-8 , p. 374 ( limited preview in Google Book search).
- ↑ by Julio Alvarez-Builla: Modern Heterocyclic Chemistry - Julio Alvarez-Builla . John Wiley & Sons, 2011, ISBN 978-3-527-33201-4 , pp. 995 ( limited preview in Google Book Search).
- ↑ Thomas M. Klapötke , Thomas Schütt: SYNTHESIS AND SPECTROSCOPIC CHARACTERIZATION OF BERYLLIUM AZIDE AND TWO DERIVATIVES. In: Main Group Metal Chemistry. 22, 1999, S., doi : 10.1515 / MGMC.1999.22.6.357 .




