Triphenyl germanium chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
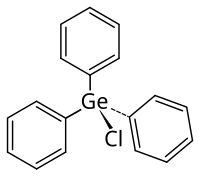
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triphenyl germanium chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 15 ClGe | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 339.4 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
114-115 ° C |
|||||||||||||||
| boiling point |
285 ° C (16 hPa ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Triphenylgermanium chloride is a chemical compound from the group of organic germanium compounds .
Extraction and presentation
Triphenyl germanium chloride can be obtained by reacting tetraphenyl germanium with germanium (IV) chloride in the presence of aluminum trichloride .
properties
Triphenylgermanium chloride is a white solid.
use
Triphenylgermanium hydride can be obtained by reacting with zinc dust in ethanol .
Individual evidence
- ↑ a b c d e f g data sheet Triphenylgermanium chloride, 99% from Sigma-Aldrich , accessed on October 7, 2017 ( PDF ).
- ↑ a b Jane E. Macintyre: Dictionary of Organometallic Compounds . CRC Press, 1994, ISBN 978-0-412-43060-2 , pp. 1742 ( limited preview in Google Book search).
