Venetoclax
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
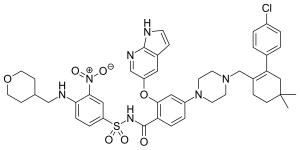
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Venetoclax | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 45 H 50 ClN 7 O 7 S | ||||||||||||||||||
| Brief description |
Light to dark yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class |
Antineoplastic agents |
||||||||||||||||||
| Mechanism of action |
Inhibition of Bcl-2 |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 868.44 g · mol -1 | ||||||||||||||||||
| solubility |
Very low solubility in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Venetoclax is a drug used to treat blood cancer . It is the first member of the class of Bcl-2 inhibitors and is orally effective.
Mechanism of action
Venetoclax selectively inhibits the protein Bcl-2 ( B-cell lymphoma 2 ), which is overexpressed in chronic lymphocytic leukemia (CLL), non-Hodgkin's lymphoma (NHL) and other B-cell lymphomas . Bcl-2 is involved in suppressing the natural process of programmed cell death ( apoptosis ). Blocking Bcl-2 restores the signaling cascade that causes cancer cells to self-destruct. Venetoclax is also able to reduce the insensitivity of Bcl-2 proteins to conventional chemotherapeutic agents , so that these can be more effective.
Pharmacokinetics
After oral administration, maximum plasma concentrations occur after 5 to 7 hours. Venetoclax is almost completely (over 99.9%) bound to plasma proteins. The main metabolism is via CYP 3A4 / 3A5 . More than 99.9% of it is excreted (of which 20.8% unchanged as venetoclax) via the faeces .
The terminal half-life is approximately 26 hours.
application areas
Venetoclax has been approved in the USA since April 2016 as Venclexta for oral therapy in patients with previously treated ( relapsed or refractory) CLL with deletion of chromosome 17p . Approval was granted through an accelerated process (“Break Through Therapy”). Approval in the European Union was granted on December 5, 2016.
The treatment of other forms of blood cancer such as indolent non-Hodgkin lymphoma, diffuse large B-cell lymphoma (DLBCL) and acute myeloid leukemia (AML) is being investigated in studies. Venetoclax has been granted orphan drug status in the EU for the treatment of AML .
Admission Studies
Study M13-982: The basis for the approval of Venclexta in the USA is an open, single-arm, multicenter phase II study in 106 CLL patients with a diagnosed 17p deletion who had previously received at least one therapy. The study participants received a dose of venetoclax once a day, which was increased from 20 mg to 400 mg over five weeks. The primary endpoint of the study was the overall response rate based on the guidelines of the National Cancer Institute-sponsored Working Group (as updated by the International Workshop for Chronic Lymphocytic Leukemia (IWCLL)). An independent committee found an overall response rate of 79.4%.
Side effects
The most common side effects observed were neutropenia , diarrhea, nausea, upper respiratory tract infections, and fatigue. Tumor lysis syndrome (TLS) was observed in five patients, but none of them had clinical consequences.
See also
Preparation names
Venclexta (USA), Venclyxto (EU)
Individual evidence
- ↑ a b c d e f VENCLEXTA (venetoclax) tablets, for oral use. US Full Prescribing Information . AbbVie Inc. Retrieved April 14, 2016.
- ↑ There is not yet a harmonized classification for this substance . What is shown is a label of 2 - ((1H-pyrrolo [2,3-b] pyridin-5-yl) oxy) -4- (4 - ((4'-chloro-5,5- dimethyl-3,4,5,6-tetrahydro- [1,1'-biphenyl] -2-yl) methyl) piperazin-1-yl) - N - ((3-nitro-4 - (((tetrahydro-2H -pyran-4-yl) methyl) amino) phenyl) sulfonyl) benzamide (amorphous) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 10, 2019.
- ↑ a b Roche press release: Crucial phase II study with investigational drug Venetoclax reached primary endpoint in difficult-to-treat form of chronic lymphocytic leukemia , dated August 12, 2015.
- ↑ a b Leukemia: U.S. Approval for Venetoclax , Pharmaceutical Newspaper, News, April 13, 2016.
- ↑ a b EU / 3/16/1617
- ↑ EMA: An overview of Venclyxto and why it is authorized in the EU , 2018.
- ↑ Susanne Heinzl: CLL: previously unseen response rates with venetoclax in ultra-high-risk patients , Medscape, December 14, 2015.