Eyelash serum
An eyelash serum , also called eyelash growth serum , lash booster or eyelash booster , is used to strengthen the natural growth of the eyelashes and thus to help people with sparse eyelashes to have a more voluminous lash line and longer eyelashes. These products to be applied to the edge of the eyelid contain not only active ingredients that promote lash growth, but also care substances that strengthen the health of the individual lashes and anchor them more firmly in the roots.
With the discovery of the eyelash growth-promoting properties of certain prostaglandin derivatives , corresponding products quickly became popular. Because they are located in the gray area between cosmetics and pharmaceuticals , they are viewed critically from the point of view of consumer protection and have also gained media attention.
Physiology of eyelash growth
The eyelash growth follows a cycle of growth, transition and rest phase . The growth phase (anagen phase) of the eyelash in humans only lasts about 1 to 2 months and is thus significantly shorter than that of the scalp hair. During the growth phase, the eyelashes grow an average of 0.15 mm daily and reach their maximum length at the end. This is followed by an approximately 15-day transition phase (catagen phase) in which the epithelial cells of the hair follicle die off ( apoptosis ). The final resting phase lasts for 4 to 9 months in the case of eyelash hair and is therefore considerably longer than that of the scalp hair. The complete cycle for human eyelashes takes about 5 to 12 months. After falling out, eyelash hairs are replaced by newly growing eyelashes.
Active ingredients
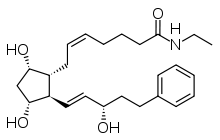
Prostaglandin derivatives
The so-called prostaglandin analogs are counted among the active substances, substances structurally related to prostaglandin F 2α , but their use in cosmetics is not uncontroversial among experts. Prostaglandins belong to the tissue hormones , i.e. local information carriers that transport the information content of external signals within the cells.
The discovery of the properties of prostaglandin analogues that promote eyelash growth can be traced back to a finding from ophthalmology and was first described in 1997 for the glaucoma drug latanoprost . Glaucoma patients who received travoprost or bimatoprost eye drops to reduce intraocular pressure ( Travatan, Lumigan ) noticed increased eyelash growth as a side effect within a few weeks. The pharmaceutical company Allergan then launched the prescription drug Latisse on the US market in 2008, which was approved by the US Food and Drug Administration for the treatment of patients with eyelash growth disorders ( hypotrichosis ). In the clinical study , a significantly improved eyelash growth compared to placebo could be demonstrated. In the double-blind study, the prominence of the lash line was assessed after treatment with a 0.03% bimatoprost solution and the length, thickness and color of the eyelash hairs were measured.
The effect of Bimatoprost is based on the lengthening of the anagen phase so that the eyelashes grow longer. Also, more eyelashes appear at the same time. In addition, the stimulation of melanogenesis induced by bimatoprost appears to lead to darker eyelashes. The exact mechanism of action is not known. Bimatoprost is a synthetically produced compound which, although structurally similar to prostaglandin F 2α , does not act via the known prostaglandin receptors. Rather, it mimics the effects of the body's own substances, the so-called prostamids , which are also involved in regulating intraocular pressure. The structure of the prostamide receptor has not yet been identified.
With the discovery of the lash growth promoting properties of certain prostaglandin analogs, eyelash growth serums quickly became popular. Latisse is not approved in European countries . Instead, there are cosmetic preparations with ingredients that are supposed to mimic the effects of bimatoprost. There are also synthetically produced prostaglandin derivatives such as:
- Cloprosten isopropyl ester
- Norbimatoprost (methylamidodihydronoralfaprostal, MDN)
- Ethyltafluprostamide ("Dechloro dihydroxy difluoro ethylcloprostenolamide")
Because of the structural relationship to bimatoprost, similar (desirable and undesirable) effects are considered likely, but there is no valid clinical documentation (studies). In Europe, a cosmetic eyelash growth product with a bimatoprost derivative came onto the market for the first time in 2009, and in 2015 it achieved sales of 13 million euros.
Other ingredients
The idea behind using alternative substances to improve eyelash growth is that they provide moisture and nutrients that hair needs for healthy, vigorous growth, and delay the aging process of the eyelash hair and roots.
Tried and tested cosmetic ingredients include: B .:
- Hyaluronic acid , panthenol and glycerine for the supply of moisture
- Caffeine to dilate blood vessels and improve the supply of nutrients
- Proteins or peptides and amino acids to strengthen the hair structure
- Vitamins such as B. Vitamin E ( tocopherol ) as an "anti-aging" agent or biotin to promote cell metabolism
- Castor oil is traditionally used as an eyelash care substance
type of application
The eyelash growth serums are applied to the cleaned upper lash line like an eyeliner for the period specified for the specific product, avoiding application to the lower edge of the eye and wetting of the eye and other areas of the skin.
Side effects and restrictions on use
Eyelash growth serums, especially those containing prostaglandin analogues, can cause a number of undesirable effects such as burning, itching, redness and swelling at the application site, as well as dry eyes, dark circles and shadows, increased blood flow to the conjunctiva ( hyperemia ), increased tear flow and eyelid inflammation . In direct contact with the eyes, bimatoprost can also cause short-term visual disturbances, change the intraocular pressure and lead to irreversible discoloration of the iris .
Since a systemic pharmacological effect cannot be ruled out, eyelash preparations with prostaglandin analogues should not be used in pregnant and breastfeeding women, children and adolescents.
criticism
Eyelash growth serums are sold as cosmetics, which is viewed critically in the case of preparations containing prostaglandin because of their pharmacological effectiveness under consumer protection aspects. According to the European Cosmetics Regulation, cosmetics must "be safe for human health when used normally or reasonably foreseeable", but unlike pharmaceuticals, for which studies on efficacy, safety and pharmaceutical quality must meet clear requirements, manufacturers of cosmetic products can use the test methods largely self-determined and do not have to make the test results public.
The Commission for Cosmetic Products of the Federal Institute for Risk Assessment (BfR), which is responsible for assessing the health risks of cosmetics in Germany, dealt with eyelash waxers containing prostglandin as early as 2011 and criticized their safety assessment. In 2018, the BfR Commission recommended that the EU Commission's Scientific Expert Committee ( SCCS ) be entrusted with the question of the safety of use of prostaglandins, prostaglandin analogues and prostamids, with the aim of banning cosmetic products. She also advocated taking substances for which negative safety assessments were already available from the BfR or comparable institutions from the market via a RAPEX notification. The Bavarian State Office for Health and Food Safety last issued a warning in 2017 against eyelash growth serums containing prostagland and advised against their use. These are functional medicinal products that are subject to mandatory approval by the higher federal authority.
The weekly magazine “ Stern ” reported that in Sweden the drug authority has been banning products with prostaglandin derivatives from the cosmetics market since 2012. The authority classifies prostaglandin analogs as active pharmaceutical ingredients.
The Dutch radio station " Avrotros " reported that the Dutch Food and Product Safety Authority (NVWA) had banned a provider from selling an eyelash preparation containing bimatoprost in 2018. In previous years, cosmetic manufacturers had already withdrawn products from the market or switched them over to the EU Commission's rapid warning system because of warnings about bimatoprost in cosmetic eyelash preparations.
In 2011, the US Food and Drug Administration (FDA) warned against the use of cloprostene isopropyl ester eyelash waxers because it found it unsafe to use.
The Canadian health authorities have banned prostaglandins and derivatives as cosmetic ingredients in their “Cosmetic Ingredient Hotlist” since 2015.
Individual evidence
- ↑ M. Lenze-Schulten: Beautiful suffering , FAZ, August 8, 2017.
- ↑ a b c N. Simon: On the hype of eyelash serum - and the risks Stern, May 22, 2017.
- ↑ a b c d e JL Cohen: Enhancing the growth of natural eyelashes: the mechanism of bimatoprost-induced eyelash growth. Dermacol Surg. 2010 Sep; 36 (9): 1361-71. doi: 10.1111 / j.1524-4725.2010.01522.x .
- ↑ T. Schlote, U. Kellner (Ed.): Unwanted drug effects in ophthalmology . Thieme Verlag, Stuttgart 2011, p. 15.
- ↑ M. Unger: Focus on the eye . Pharmaceutical newspaper, issue 10, 2003.
- ^ D. Jones: Enhanced Eyelashes: Prescription and Over-the-Counter. Options. Aesthetic Plast Surg. 2011 Feb; 35 (1): 116-121. doi: 10.1007 / s00266-010-9561-3
- ^ S. Smith et al .: Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J Am Acad Dermatol. 2012 May; 66 (5): 801-6. doi: 10.1016 / j.jaad.2011.06.005 .
- ↑ a b Prescribing information Latisse® (bimatoprost ophthalmic solution) 0.03% NDA 22-369, FDA, December 2008.
- ↑ Mutschler drug effects , 10th edition, Wissenschaftl. Verlagsgesellschaft mbH Stuttgart, p. 673, ISBN 978-3-8047-2898-1 .
- ↑ Scott D Smid: Role of prostaglandins and specific place in therapy of bimatoprost in the treatment of elevated intraocular pressure and ocular hypertension: A closer look at the agonist properties of bimatoprost and the prostamides . Clin Ophthalmol. 2009; 3: 663-670. PMID 20054414 . PMC 2801635 (free full text).
- ↑ a b c Långa ögonfransar - snygg till vilket pris ("Long eyelashes - good looking at any price"). Information from the Swedish Medicines Agency, Issue 24, September 2013 (Swedish)
- ↑ Claudia Büdel: Eyelash Serums - An Attack on Eye Health? DOZ-Verlag optical specialist publication, accessed on February 21, 2019 .
- ↑ Isopropyl Cloprostenate in the CosIng database
- ↑ Norbimatoprost in the CosIng database
- ↑ Ethyltafluprostamide in the CosIng database
- ↑ Eyelash serums - the perfect look ... Prostaglandins as eyelash growth agents , The Cosmetic Experts of the Chemical and Veterinary Investigation Office (CVUA) Karlsruhe, July 16, 2018.
- ↑ a b c d C. Wendt: Open your eyes to prostaglandin serums pta forum issue 24, 2017. Avoxa - Mediengruppe Deutscher Apotheker GmbH.
- ↑ TW Fischer, UC Hipler, P. Elsner: Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. International Journal of Dermatology . 46, 2007, pp. 27-35. PMID 17214716 .
- ↑ Lumigan: Summary of Product Characteristics ( Summary of Product Characteristics ), as of October 2018.
- ↑ Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of November 30, 2009 on cosmetic products
- ↑ 8th meeting of the BfR Commission for Cosmetic Products - minutes of the meeting on November 23, 2011 , p. 5. Retrieved on February 21, 2019.
- ↑ 21st meeting of the BfR Commission for Cosmetic Products - Protocol of April 18, 2018 , p. 2. Accessed on February 21, 2019.
- ↑ warning of prostaglandin-containing Wimpernwachstumsseren , Bavarian State Office for Health and Food Safety on 12 April 2017th
- ↑ a b Ook risico bij wimperserums zonder het geneesmiddel Bimatoprost ("Risk even with eyelash serums without the drug Bimatoprost") AVROTROS , 10 September 2018 (Dutch).
- ↑ Safety Gate: the rapid alert system for dangerous non-food products
- ^ FDA, US Food and Drug Administration, Warning Letter, LLC 4/18/11.
- ↑ List of Ingredients that are Prohibited for Use in Cosmetic Products , accessed February 21, 2019.