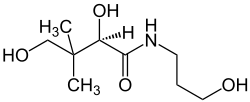
Dexpanthenol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Dexpanthenol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 9 H 19 NO 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 205.25 g · mol -1 | |||||||||||||||||||||
| density |
1.2 g cm −3 |
|||||||||||||||||||||
| boiling point | ||||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.497 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Dexpanthenol (also known as pantothenol , D-panthenol , provitamin B5 or simply panthenol ) is a chemical compound that belongs to the polyols and amides . The compound has long been used medicinally as an active ingredient in the topical ( "topical" ) treatment of diseases of the skin and mucous membranes, and cosmetically in medicinal skin care , and administered intravenously to promote intestinal activity.
Provitamin
Dexpanthenol is a provitamin ; it is converted into pantothenic acid (vitamin B5) in the body. Pantothenic acid is a component of coenzyme A and thus plays an essential role in the skin metabolism.
Analytics
Dexpanthenol is in the different preparations after an appropriate sample preparation by HPLC demonstrated techniques.
Effect and areas of application
Dexpanthenol is well absorbed by the skin in water-oil emulsions and accumulates at the place of application. It increases the skin's ability to retain moisture and thus has nourishing properties and improves the skin's elasticity. It supports the regeneration of skin cells and thus contributes to regeneration. In addition, dexpanthenol also has itching- relieving, anti-inflammatory and wound healing properties.
The active ingredient is used by many manufacturers as an ingredient in skin creams , ointments and shampoos, as well as lozenges , nasal sprays , eye drops and contact lens cleaning products. Furthermore, panthenol is used to heal new tattoos .
When administered intravenously, dexpanthenol is mainly used to treat intestinal atony (intestinal paralysis). When applied this way, it has laxative properties.
The active ingredient is also used in aquaristics, where it is used as a component of Crustacea care products and is intended to support the animals' moulting process.
Side effects
Dexpanthenol is generally very well tolerated. In very rare cases, however, irritation, redness or contact allergies can occur. Such an allergy can be detected by an allergy test , which may be repeated after eight days in order to exclude sensitization from the initial contact.
With intravenous administration, cardiac arrhythmias up to pronounced bradycardia occur as a side effect in some patients .
Trade names
Bepanthen (D, A, CH), Bepanthol, Corneregel (D, A), Marolderm (D), nasal spray Ratiopharm Panthenol (D), Nasic-cur (D), Pan-Ophtal (D), Panthenol foam spray (D, A , CH, I), various generics (D)
- Combination preparations (selection)
Addivit (D), Aspecton Nasenspray (D), Bepanthen Antiseptic Wound Cream (D), Bepanthen plus (A, CH), Bepanthen Nasensalbe (CH), Cernevit (D, A), Colda-Balsam (A), Dispatenol (D) , Doloben (D, A), Inzolen (D), Nasic (D), Pantozet (A), Siccaprotect (D, A), Venoben (A)
Individual evidence
- ↑ entry to PANTHENOL in CosIng database of the European Commission, accessed on 28 December of 2019.
- ↑ a b c d data sheet D-Panthenol from Sigma-Aldrich , accessed on July 1, 2019 ( PDF ).
- ↑ a b c Data sheet D-Panthenol, 98 +% from AlfaAesar, accessed on July 1, 2019 ( PDF )(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-138.
- ^ AK De, PP Chowdhury, S. Chattapadhyay: Simultaneous Quantification of Dexpanthenol and Resorcinol from Hair Care Formulation Using Liquid Chromatography: Method Development and Validation. , Scientifica (Cairo). 2016; 2016: 1537952. PMID 27042377 .
- ↑ AU Kulikov, AA Zinchenko: Development and validation of reversed phase high performance liquid chromatography method for determination of dexpanthenol in pharmaceutical formulations. J Pharm Biomed Anal. February 19, 2007; 43 (3): pp. 983-988, PMID 17049793 .
- ↑ a b c F. Ebner et al .: Topical use of dexpanthenol in skin disorders. In: American Journal of Clinical Dermatology . 3 (6), 2002, pp. 427-433. PMID 12113650 .
- ↑ W. Stozkowska, R. Piękoś: Investigation of some topical formula tions Containing dexpanthenol. In: Acta Pol Pharm . 61, 2004, pp. 433-437. PMID 15794335 .
- ↑ E. Proksch, HP Nissen: Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. In: J Dermatolog Treat. 13, 2002, pp. 173-178. PMID 19753737 .
- ↑ Dennerle: Fascination Nano-Aquariums Product Catalog.
