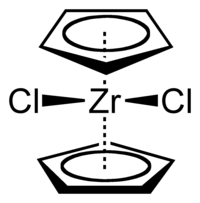
Zirconocene dichloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Zirconocene dichloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 10 Cl 2 Zr | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 292.32 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.785 g cm −3 |
|||||||||||||||
| Melting point |
242-245 ° C |
|||||||||||||||
| solubility |
soluble in organic solvents, hydrolysis in water |
|||||||||||||||
| Refractive index |
1.555 ± 0.005 at the melting temperature |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Zirconocene dichloride is an organozirconium compound consisting of a central zirconium atom as well as two cyclopentadienyl ligands and two chloride ligands. Zirconocene dichloride is a diamagnetic , white solid that is relatively stable in air.
Presentation and structure
Zirconocene dichloride can be prepared from a zirconium (IV) chloride - THF complex and cyclopentadienyl sodium :
- ZrCl 4 (THF) 2 + 2 NaCp → Cp 2 ZrCl 2 + 2 NaCl + 2 THF
The closely related compound Cp 2 ZrBr 2 was first described by Birmingham and Wilkinson.
The structure of the connection is similar to that of a folding shovel, the Cp rings are not parallel and the average Cp-M-Cp angle (from the center of the area) is 128 °. The Cl-Zr-Cl angle of 97.1 ° is wider than in niobocene dichloride (85.6 °) and molybdocene dichloride (82 °). Knowledge of this trend helped to demonstrate the orientation of the HOMO in this class of complexes.
Reactions
Zirconocene dichloride reacts with lithium aluminum hydride to form Cp 2 ZrHCl, the Schwartz reagent :
- (C 5 H 5 ) 2 ZrCl 2 + 1 / 4 LiAlH 4 → (C 5 H 5 ) 2 ZrHCl + 1 / 4 "LiAlCl 4 "
Since lithium aluminum hydride is a strong reducing agent, the dihydro complex Cp 2 ZrH 2 can be formed if the reaction is too extensive . This can be converted into Schwartz reagent by dichloromethane .
Zirconocene dichloride is also used industrially as a catalyst or precatalyst in polymerization reactions . Polymers are also accessible that are not accessible via normal Ziegler-Natta catalysts and z. B. have special tacticity , molecular weight or molecular weight distribution.
The cyclopentadienyl ligands of zirconocene dichloride can be converted into the corresponding 1,2,3,4,5-pentaaryl-1,3-cyclopentadienes, such as 1,2,3,4,5-pentakis , by multiple arylation with aryl bromides in palladium-catalyzed reactions (4-butylphenyl) -1,3-cyclopentadiene react.
literature
- A. Maureen Rouhi: Organozirconium Chemistry Arrives . In: Chemical & Engineering News . tape 82 , no. 16 , 1998, pp. 162 , doi : 10.1021 / cen-v082n015.p035 ( acs.org ).
Individual evidence
- ↑ Entry on organic zirconium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ a b c H. B. Bradley, LG Dowell: Crystallographic Data. 168. Bis (cyclopentadienyl) zirconium dichloride . In: Analytical Chemistry . tape 30 , no. 4 , 1958, pp. 548-548 , doi : 10.1021 / ac60136a601 .
- ↑ a b c data sheet bis (cyclopentadienyl) zirconium (IV) dichloride, 98% from Sigma-Aldrich , accessed on March 6, 2013 ( PDF ).
- ↑ G. Wilkinson, JM Birmingham: Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta . In: Journal of the American Chemical Society . tape 76 , no. 17 , September 1, 1954, p. 4281-4284 , doi : 10.1021 / ja01646a008 .
- ↑ K. Prout, TS Cameron, RA Ford, SR Critchley (in part), B. Denton (in part), GV Rees (in part): The crystal and molecular structures of bent bis-π-cyclopentadienyl – metal complexes: ( a) bis-π-cyclopentadienyldibromorhenium (V) tetrafluoroborate, (b) bis-π-cyclopentadienyldichloromolybdenum (IV), (c) bis-π-cyclopentadienylhydroxomethylaminomolybdenum (IV), hexafluorophosphate, (d) bis-bis-π-cycloethyl e) bis-π-cyclopentadienyldichloroniobium (IV), (f) bis-π-cyclopentadienyldichloromolybdenum (V) tetrafluoroborate, (g) μ-oxo-bis [bis-π-cyclopentadienylchloroniobium (IV)] tetrafluoroborate, (h) bis-π -cyclopentadienyldichlorozirconium . In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry . tape 30 , no. 10 , October 15, 1974, p. 2290-2304 , doi : 10.1107 / S0567740874007011 .
- ↑ SL Buchwald, SJ LaMaire, RB Nielsen, BT Watson, SM King: Schwartz's Reagent In: Organic Syntheses . 71, 1993, p. 77, doi : 10.15227 / orgsyn.071.0077 ; Coll. Vol. 9, 1998, p. 162 ( PDF ).
- ↑ Ralf Alsfasser, HJ Meyer: Moderne Anorganische Chemie . 2nd Edition. deGruyter, 2007, ISBN 978-3-11-017838-8 ( limited preview in Google book search).
- ↑ Joachim Buddrus: Fundamentals of organic chemistry . 3. Edition. deGruyter, 2003, ISBN 3-11-014683-5 ( limited preview in Google book search).
- ↑ Gerald Dyker, Jörg Heiermann, Masahiro Miura, Jun-Ichi Inoh, Sommai Pivsa-Art, Tetsuya Satoh, Masakatsu Nomura: Palladium-Catalyzed Arylation of Cyclopentadienes. In: Chemistry - A European Journal . 6, 2000, pp. 3426-3433, doi : 10.1002 / 1521-3765 (20000915) 6:18 <3426 :: AID-CHEM3426> 3.0.CO; 2-B .
