Zolmitriptan
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
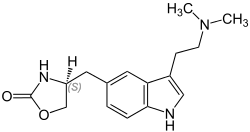
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Zolmitriptan | ||||||||||||||||||
| other names |
( S ) -4- {3- [2- (Dimethylamino) ethyl] indol-5-ylmethyl} oxazolidin-2-one |
||||||||||||||||||
| Molecular formula | C 16 H 21 N 3 O 2 | ||||||||||||||||||
| Brief description |
white to almost white, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Selective 5-HT 1 receptor agonist |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 287.36 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
139-141 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Zolmitriptan is a selective serotonin agonist from the triptan group and is used as a medicinal substance in the acute therapy of migraines . Zolmitriptan is also approved as a nasal spray for the treatment of cluster headache attacks. Zolmitriptan is subject to medical prescription .
pharmacology
Mechanism of action
Zolmitriptan is a selective agonist at the serotonin receptors 5-HT 1B and 5-HT 1D , which occur on cerebral blood vessels and presynaptically on neurons . Activation of these receptors by zolmitriptan thus leads, on the one hand, to a constriction of the dilated cerebral blood vessels during a migraine attack and, on the other hand, to a reduction in the release of inflammatory mediators .
Side effects
In addition to non-specific side effects and effects that may be attributable to the underlying disease migraine (e.g. dizziness, nausea), QTc time extensions and other cardiovascular disorders (e.g. tachycardia , rise in blood pressure) can occasionally occur. Rare side effects of the use of zolmitriptan are angina pectoris- like pressure and tightness in the chest, which are attributed to a constriction of the coronary arteries .
The Drugs Commission of the German Medical Association (AkdÄ) reported in January 2013: Peripheral arterial occlusion in connection with zolmitriptan (from the UAW database) .
Interactions
Zolmitriptan should not be used in combination with ergotamine , as there is an increased risk of coronary spasms. Inhibitors of monoamine oxidase ( MAOIs ) and the cytochrome P450 enzyme system can slow down the breakdown of zolmitriptan.
The American health authority FDA warns of the potentially life-threatening interaction of the serotonin syndrome (the body accumulates too much serotonin in the nervous system) when taking a triptan and an antidepressant from the group of SSRIs (selective serotonin reuptake inhibitors) or SNRI (selective serotonin inhibitors) at the same time. and norepinephrine reuptake inhibitors) when triptans are taken with an SSRI or an SNRI. Symptoms of serotonin syndrome can include restlessness, hallucinations, loss of coordination, rapid heartbeat, fluctuations in blood pressure, increased body temperature, increased reflexes, nausea, vomiting, and diarrhea.
chemistry
Zolmitriptan belongs to the group of triptans . It is a further development of sumatriptan with modified pharmacokinetic properties. In particular, the molecule is less polar so that it is better absorbed from the intestine and better penetrates the blood-brain barrier .
Individual evidence
- ↑ a b Label for Zolmitriptan ZOMIG ® Tablets and ZOMIG-ZMT ® - FDA label August 11, 2010 (PDF; 161 kB).
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1754, ISBN 978-0-911910-00-1 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of (4R) -4 - [[3- (2-dimethylaminoethyl) -1H-indol-5-yl] methyl] oxazolidin-2-ones in the Classification and Labeling Inventory of the European Chemicals Agency is reproduced from a self-classification by the distributor (ECHA), accessed on July 12, 2020.
- ↑ Limmroth V: mechanism of action of triptans . In: Pharmacy in our time . 31, No. 5, 2002, pp. 458-461. doi : 10.1002 / 1615-1003 (200209) 31: 5 <458 :: AID-PAUZ458> 3.0.CO; 2-G . PMID 12369163 .
- ↑ Torsten Kratz, Albert Diefenbacher: Psychopharmacotherapy in old age. Avoidance of drug interactions and polypharmacy. In: Deutsches Ärzteblatt. Volume 116, Issue 29 f. (July 22) 2019, pp. 508-517, p. 512.
- ↑ Peripheral arterial occlusion in connection with zolmitriptan , AkdÄ announcements, accessed on January 7, 2013.
- ↑ Peripheral arterial occlusion in connection with zolmitriptan (PDF; 292 kB), publication DÄ, 110, 1-2, January 7, 2013.
- ↑ FDA Public Health Advisory: Combined Use of 5-Hydroxytryptamine Receptor Agonists (Triptans), Selective Serotonin Reuptake Inhibitors (SSRIs) or Selective Serotonin / Norepinephrine Reuptake Inhibitors (SNRIs) May Result in Life-threatening Serotonin Syndrome , July 19, 2006 ( English).