Pyrylium: Difference between revisions
m Task 18 (cosmetic): eval 9 templates: del empty params (1×); |
CrafterNova (talk | contribs) →Derivatives: corrected spelling |
||
| (23 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| |
|Verifiedfields = changed |
||
| |
|Watchedfields = changed |
||
| |
|verifiedrevid = 443022610 |
||
| |
|Name=Pyrylium |
||
| |
|ImageFileL1 = Pyrylium.svg |
||
| |
|ImageFileR1 = Pyrylium-3D-balls.png |
||
|PIN = Pyrylium<ref>{{cite book |author=International Union of Pure and Applied Chemistry |author-link=International Union of Pure and Applied Chemistry |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=[[Royal Society of Chemistry|The Royal Society of Chemistry]] |page=1097 |doi=10.1039/9781849733069 |isbn=978-0-85404-182-4}}</ref> |
|||
| IUPACName = Pyrylium |
|||
| |
|OtherNames = Pyranium |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| |
|CASNo_Ref = {{cascite|correct|??}} |
||
| index1_label=salt |
|||
| ⚫ | |||
| index2_label=family |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| |
| Beilstein = 1421881 |
||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ⚫ | |||
| ⚫ | |||
|ChEBI = 36120 |
|||
| ⚫ | |||
|ChEBI1 = 59658 |
|||
| ⚫ | |||
|ChEBI2 = 59657 |
|||
| ⚫ | |||
| |
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ⚫ | |||
| ⚫ | |||
| Gmelin = 558560 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| |
|Formula=C<sub>5</sub>H<sub>5</sub>O<sup>+</sup> |
||
| |
|MolarMass=81.09 g/mol |
||
| Appearance= |
|||
| Density= |
|||
| MeltingPt= |
|||
| BoilingPt= |
|||
| Solubility= |
|||
| ⚫ | |||
| ⚫ | |||
| MainHazards= |
|||
| FlashPt= |
|||
| AutoignitionPt = |
|||
}} |
}} |
||
| ⚫ | |||
| OtherCompounds = [[thiopyrylium]], [[selenopyrylium]], [[telluropyrylium]] |
|||
| ⚫ | |||
}} |
}} |
||
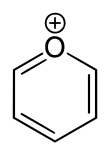
'''Pyrylium''' is a [[cation]] (positive [[ion]]) with formula {{ |
'''Pyrylium''' is a [[cation]] (positive [[ion]]) with formula {{chem2|C5H5O+}}, consisting of a six-membered ring of five [[carbon]] atoms, each with one [[hydrogen]] atom, and one positively charged [[oxygen]] atom. The bonds in the ring are [[conjugated system|conjugated]] as in [[benzene]], giving it an [[aromaticity|aromatic]] character. In particular, because of the positive charge, the oxygen atom is [[valence (chemistry)|trivalent]]. Pyrilium is a mono-[[cyclic compound|cyclic]] and [[heterocyclic compound|heterocyclic]] compound, one of the [[oxonium ion]]s. |
||
==Salts== |
==Salts== |
||
Pyrylium and its derivatives form stable [[salt (chemistry)|salt]]s with a variety of anions.<ref>{{cite book |title= Heterocyclic Chemistry |author= Gilchrist, T. L. |isbn= 0-582-27843-0}}</ref><ref>{{cite |
Pyrylium and its derivatives form stable [[salt (chemistry)|salt]]s with a variety of anions.<ref>{{cite book |title= Heterocyclic Chemistry |author= Gilchrist, T. L. |year= 1997 |isbn= 0-582-27843-0}}</ref><ref>{{cite book |author1=Balaban, A. T. |author2=Schroth, W. |author3=Fischer, G. |title= Pyrylium Salts. I. Synthesis |series= Advances in Heterocyclic Chemistry |editor1=Katritzky, A. R. |editor2=Boulton, A. J. |publisher= Academic Press |location= New York |year= 1969 |volume= 10 |pages= 241–326 |doi= 10.1016/S0065-2725(08)60499-7}}</ref><ref>{{cite book |author1=Balaban, A. T. |author2=Dinculescu, A. |author3=Dorofeenko, G. N. |author4=Fischer, G. W. |author5=Koblik, A. V. |author6=Mezheritskii, V. V. |author7=Schroth, W. |title= Pyrylium Salts. Syntheses, Reactions and Physical Properties |series= Advances in Heterocyclic Chemistry: Supplement |volume= 2 |editor= Katritzky, A. R. |publisher= Academic Press |location= New York |year= 1982 |isbn= 978-0-12-020652-0}}</ref><ref>{{cite book |author= Balaban, A. T. |chapter= The Pyrylium Cation as a Synthon in Organic Chemistry |title= New Trends in Heterocyclic Chemistry |editor1= Mitra, R. B. |editor2= Ayyangar, N. R. |editor3= Gogte, V. N. |editor4= Acheson, R. M. |editor5= Cromwell, N. |publisher= Elsevier |location= Amsterdam |year= 1979 |pages= [https://archive.org/details/comprehensivecar0000unse/page/79 79–111] |isbn= 978-0-444-41737-4 |series= Studies in Organic Chemistry |volume= 3 |chapter-url= https://archive.org/details/comprehensivecar0000unse/page/79 }}</ref><ref>{{cite book |author= Balaban, A. T. |chapter= Pyrylium Salts as Useful Synthons |title= Organic Synthesis: Modern Trends |editor= Chizov, O. |publisher= Blackwell |location= Oxford |year= 1987 |pages= 263–274 |isbn= 0-632-02014-8}}</ref><ref>{{cite book |author1=Balaban, T. S. |author2=Balaban, A. T. |chapter= Pyrylium Salts |series= Science of Synthesis; Houben-Weyl Methods of Molecular Transformations |publisher= Georg Thieme Verlag |location= Stuttgart |year= 2003 |volume= 14 |title= Hetarenes and Related Ring Systems, Six-membered Hetarenes with one Chalcogen |pages= 11–200 |isbn= 978-3-13-118641-6}}</ref> |
||
<!---Pyrylium [[perchlorate]] is soluble in [[acetonitrile]]. |
<!---Pyrylium [[perchlorate]] is soluble in [[acetonitrile]]. |
||
Trimethylpyrylium perchlorate has a melting point of |
Trimethylpyrylium perchlorate has a melting point of 246–247 °C. |
||
2,4,6-Triphenylpyrylium tetrafluoroborate, yellow needles, mp 214-215° |
2,4,6-Triphenylpyrylium tetrafluoroborate, yellow needles, mp 214-215° |
||
2-Methyl-4,6-diphenyl pyrylium chloride: light yellow, mp |
2-Methyl-4,6-diphenyl pyrylium chloride: light yellow, mp 125–126.--> |
||
==Derivatives== |
==Derivatives== |
||
Many important cations are formally derived from pyrylium by substitution of various [[functional group]]s for some or all the hydrogens in the ring. The [[2,4,6- |
Many important cations are formally derived from pyrylium by substitution of various [[functional group]]s for some or all the hydrogens in the ring. The [[2,4,6-triphenylpyrylium]], referred to as the Katritzky salt, (after [[Alan R. Katritzky]]) is an important example used in many modern examples of metal catalyzed [[Cross-coupling reaction|cross-couplings]].<ref name=bala1977>{{cite journal|first1=A. T.|last1=Balaban|first2=V.|last2=Wray|date=1977|title=<sup>13</sup>C n.m.r. spectra of some pyrylium salts and related compounds|journal=Organic Magnetic Resonance|volume=9|issue=1|pages=16–22|doi=10.1002/mrc.1270090105}} |
||
</ref> |
</ref> |
||
==Chemical properties== |
==Chemical properties== |
||
Like other [[oxonium ion]]s, pyrylium is unstable in neutral water. However, pyrylium is much less reactive than ordinary oxonium ions because of aromatic stabilization. The 2,4,6-triphenyl salt is commonly reacted with [[Aliphatic compound|aliphatic]] [[ |
Like other [[oxonium ion]]s, pyrylium is unstable in neutral water. However, pyrylium is much less reactive than ordinary oxonium ions because of aromatic stabilization. The 2,4,6-triphenyl salt is commonly reacted with [[Aliphatic compound|aliphatic]] [[amine]]s at the 1 position, forming [[pyridinium]] salts and activating them towards [[oxidative addition]] by metal complexes, most notably ones with [[nickel]].<ref>{{Cite journal|last1=Pang|first1=Yue|last2=Moser|first2=Daniel|last3=Cornella|first3=Josep|date=2020|title=Pyrylium Salts: Selective Reagents for the Activation of Primary Amino Groups in Organic Synthesis|url=https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0039-1690703|journal=Synthesis|volume=52|issue=4|pages=489–503|doi=10.1055/s-0039-1690703|s2cid=208705148}}</ref> Pyrylium cations also react with [[nucleophile]]s in the 2, 4, and 6 positions, which can induce a variety of reactions. The high electronegativity of the oxygen results in strong single perturbation by one [[heteroatom]] in the six-membered ring. |
||
==Synthesis== |
==Synthesis== |
||
Pyrylium salts are easily produced from simple starting materials through a [[condensation |
Pyrylium salts are easily produced from simple starting materials through a [[condensation reaction]]. |
||
Pyrylium salts with aromatic substituents, such 2,4,6-triphenylpyrylium tetrafluoroborate, can be obtained from two moles of [[acetophenone]] and one mole of [[benzaldehyde]] in the presence of [[tetrafluoroboric acid]] and an oxidizing agent (Dilthey synthesis). For pyrylium salts with alkyl substituents, such as 2,4,6-trimethylpyrylium salts, the best method uses the [[Alexandru Balaban|Balaban]]-[[Costin Nenițescu|Nenitzescu]]-Praill synthesis from [[ |
Pyrylium salts with aromatic substituents, such 2,4,6-triphenylpyrylium tetrafluoroborate, can be obtained from two moles of [[acetophenone]] and one mole of [[benzaldehyde]] in the presence of [[tetrafluoroboric acid]] and an oxidizing agent (Dilthey synthesis). For pyrylium salts with alkyl substituents, such as 2,4,6-trimethylpyrylium salts, the best method uses the [[Alexandru Balaban|Balaban]]-[[Costin Nenițescu|Nenitzescu]]-Praill synthesis from [[tertiary butanol]] and [[acetic anhydride]] in the presence of tetrafluoroboric, perchloric, or trifluoromethanesulfonic acids.<ref>{{OrgSynth |author= Balaban, A. T. |author2= Boulton, A. J. |collvol= 5 |collvolpages= 1112–1113 |year= 1973 |title= 2,4,6-Trimethyl-Pyrylium Tetrafluoroborate |prep= cv5p1112 |url= http://www.orgsyn.org/orgsyn/pdfs/CV5P1112.pdf}}</ref><ref>{{OrgSynth |author= Balaban, A. T. |author2= Boulton, A. J. |collvol= 5 |collvolpages= 1114–1116 |year= 1973 |title= 2,4,6-Trimethyl-Pyrylium Trifluoromethanesulfonate |prep= cv51114 |url= http://www.orgsyn.org/orgsyn/pdfs/CV5P1114.pdf}}</ref> 2,4,6-Triphenylpyrylium salts are converted by bases into a stable 1,5-enedione (pseudobase), but 2,4,6-trimethylpyrylium salts on treatment with hot alkali hydroxides afford an unstable pseudobase that undergoes an intramolecular condensation yielding 3,5-[[xylenol|dimethylphenol]]. In warm deuterium oxide, 2,4,6-trimethylpyrylium salts undergo isotopic exchange of 4-methyl hydrogens faster than for the 2- and 6-methyl groups, allowing the synthesis of regioselectively deuterated compounds. |
||
==Derivatives== |
==Derivatives== |
||
The reactivity of pyrylium salts toward nucleophiles makes them useful materials for producing other compounds with stronger aromatic character. Pyrylium salts afford [[pyridine]]s with [[ammonia]],<ref>{{OrgSynth |author= Anderson, A. G. |
The reactivity of pyrylium salts toward nucleophiles makes them useful materials for producing other compounds with stronger aromatic character. Pyrylium salts afford [[pyridine]]s with [[ammonia]],<ref>{{OrgSynth |author= Anderson, A. G. |author2= Stang, P. J. |title= 2,6-Di-''tert''-Butyl-4-Methylpyridine |collvol= 7 |collvolpages= 144 |year= 1981 |volume= 60 |pages= 34 |prep= cv7p0144 |url= http://www.orgsyn.org/orgsyn/pdfs/CV7P0144.pdf}}</ref> [[pyridinium]] salts with primary amines, [[pyridine-N-oxide|pyridine-''N''-oxides]] with [[hydroxylamine]], [[phosphabenzene]]s with [[phosphine]] derivatives, [[thiopyrylium]] salts with [[hydrogen sulfide]], and benzene derivatives with [[acetonitrile]] or [[nitromethane]]. |
||
==Pyrones== |
==Pyrones== |
||
[[File:Pyrones.png|210 px|right|pyrones]] |
[[File:Pyrones.png|210 px|right|pyrones]] |
||
A pyrylium cation with a [[hydroxyl]] anion [[substituent]] in the 2-position is not the [[zwitterion]]ic aromatic compound ('''1'''), but |
A pyrylium cation with a [[hydroxyl]] anion [[substituent]] in the 2-position is not the [[zwitterion]]ic aromatic compound ('''1'''), but the neutral [[Saturated and unsaturated compounds|unsaturated]] [[lactone]] [[2-pyrone]] or pyran-2-one ('''2'''). Important representatives of this class are the [[coumarin]]s. Likewise a 4-hydroxyl pyrylium compound is a [[γ-pyrone]] or pyran-4-one ('''4'''), to which group belong compounds such as [[maltol]]. |
||
===Chemical properties=== |
===Chemical properties=== |
||
[[File:Pyronecycloaddition.png|400px|center|Pyrone cycloaddition]] |
[[File:Pyronecycloaddition.png|400px|center|Pyrone cycloaddition]] |
||
2-Pyrones are known to react with [[alkyne]]s in a [[Diels–Alder reaction]] to form [[arene]] compounds with expulsion of [[carbon dioxide]], for example:<ref>{{cite journal |author1=Delaney, P. M. |author2=Moore, J. E. |author3=Harrity, J. P. A. |title= An Alkynylboronic Ester Cycloaddition Route to Functionalised Aromatic Boronic Esters |journal= [[Chemical Communications]] |year= 2006 |volume= 2006 |issue= 31 |pages= 3323–3325 |doi= 10.1039/b607322k}}</ref> |
2-Pyrones are known to react with [[alkyne]]s in a [[Diels–Alder reaction]] to form [[arene]] compounds with expulsion of [[carbon dioxide]], for example:<ref>{{cite journal |author1=Delaney, P. M. |author2=Moore, J. E. |author3=Harrity, J. P. A. |title= An Alkynylboronic Ester Cycloaddition Route to Functionalised Aromatic Boronic Esters |journal= [[Chemical Communications]] |year= 2006 |volume= 2006 |issue= 31 |pages= 3323–3325 |doi= 10.1039/b607322k|pmid=16883424 }}</ref> |
||
==Polycyclic pyrylium ions== |
==Polycyclic pyrylium ions== |
||
===Chromenylium ion=== |
===Chromenylium ion=== |
||
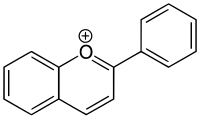
One [[bicyclic molecule|bicyclic]] pyrylium ion is called benzopyrylium ion ([[IUPAC]]: chromenylium ion) ( |
One [[bicyclic molecule|bicyclic]] pyrylium ion is called benzopyrylium ion ([[IUPAC]]: chromenylium ion) (formula: {{Chem2|C9H7O+}}, molar mass: 131.15 g/mol, exact mass: 131.04968983). It can be seen as a charged derivative of 2''H''-1-[[benzopyran]] (IUPAC: 2''H''-chromene, {{Chem2|C9H8O}}), or a (charged) substituted heterocyclic derivative of [[naphthalene]] ({{Chem2|C10H8}}). |
||
===Flavylium ion=== |
===Flavylium ion=== |
||
In biology, the 2-phenylbenzopyrylium (2-phenylchromenylium) ion is referred to as flavylium. A class of flavylium-derived compounds are [[anthocyanidin]]s and [[anthocyanin]]s, pigments that are responsible for the colors of many flowers. |
In biology, the 2-phenylbenzopyrylium (2-phenylchromenylium) ion is referred to as flavylium. A class of flavylium-derived compounds are [[anthocyanidin]]s and [[anthocyanin]]s, pigments that are responsible for the colors of many flowers.{{Citation needed|date=July 2022}} |
||
===Naphthoxanthenium cation=== |
===Naphthoxanthenium cation=== |
||
Higher [[polycyclic compound|polycyclic]] derivatives of pyrylium also exist. One good example is naphthoxanthenium. This dye is highly stable, aromatic, and planar. It absorbs in the UV and blue region and presents exceptional photophysical properties. It can be synthesized by chemical or photochemical reactions.<ref>{{cite journal |author1=Bucher, G. |author2=Bresolí-Obach, R. |author3=Brosa, C. |author4=Flors, C. |author5=Luis, J. L. |author6=Grillo, T. A. |author7=Nonell, S. |title= β-Phenyl quenching of 9-phenylphenalenones: a novel photocyclisation reaction with biological implications |journal= [[Physical Chemistry Chemical Physics]] |year= 2014 |volume= 16 |pages= 18813–18820 |doi= 10.1039/C4CP02783C|bibcode=2014PCCP...1618813B }}</ref> |
Higher [[polycyclic compound|polycyclic]] derivatives of pyrylium also exist. One good example is [[naphthoxanthenium]]. This dye is highly stable, aromatic, and planar. It absorbs in the UV and blue region and presents exceptional photophysical properties. It can be synthesized by chemical or photochemical reactions.<ref>{{cite journal |author1=Bucher, G. |author2=Bresolí-Obach, R. |author3=Brosa, C. |author4=Flors, C. |author5=Luis, J. L. |author6=Grillo, T. A. |author7=Nonell, S. |title= β-Phenyl quenching of 9-phenylphenalenones: a novel photocyclisation reaction with biological implications |journal= [[Physical Chemistry Chemical Physics]] |year= 2014 |volume= 16 |issue=35 |pages= 18813–18820 |doi= 10.1039/C4CP02783C|pmid=25079707 |bibcode=2014PCCP...1618813B }}</ref> |
||
<gallery widths=200px> |
<gallery widths=200px> |
||
File:Benzopyryliumchlorid.svg|Benzopyrylium chloride (chromenylium chloride), a salt with [[chloride]] as the [[counterion]] |
File:Benzopyryliumchlorid.svg|Benzopyrylium chloride (chromenylium chloride), a salt with [[chloride]] as the [[counterion]] |
||
File:Flavylium cation.svg|Flavylium cation |
File:Flavylium cation.svg|Flavylium cation |
||
File:Naphthoxanthenium. |
File:Naphthoxanthenium.svg|Naphthoxanthenium cation |
||
</gallery> |
</gallery> |
||
==See also== |
==See also== |
||
| ⚫ | |||
* 6-membered aromatic rings with one carbon replaced by another group:[[borabenzene]], [[silabenzene]], [[germabenzene]], [[stannabenzene]], [[pyridine]], [[phosphorine]], [[arsabenzene]], [[stibabenzene]], [[bismabenzene]], [[pyrylium]], [[thiopyrylium]], [[selenopyrylium]], [[telluropyrylium]] |
* 6-membered aromatic rings with one carbon replaced by another group: [[borabenzene]], [[silabenzene]], [[germabenzene]], [[stannabenzene]], [[pyridine]], [[phosphorine]], [[arsabenzene]], [[stibabenzene]], [[bismabenzene]], [[pyrylium]], [[thiopyrylium]], [[selenopyrylium]], [[telluropyrylium]] |
||
*[[Pyran]], {{ |
*[[Pyran]], {{Chem2|C5H6O}} (pyrones lacking the ketone group) |
||
| ⚫ | |||
==References== |
==References== |
||
{{ |
{{Reflist}} |
||
{{DEFAULTSORT:Pyrylium |
{{DEFAULTSORT:Pyrylium salt}} |
||
[[Category:Oxygen heterocycles]] |
[[Category:Oxygen heterocycles]] |
||
[[Category:Six-membered rings]] |
[[Category:Six-membered rings]] |
||
[[Category:Cations]] |
|||
[[Category:Oxonium compounds]] |
|||
Latest revision as of 07:02, 4 February 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyrylium[1] | |||
| Other names
Pyranium
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1421881 | |||
| ChEBI |
| ||
| ChemSpider | |||
| 558560 | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H5O+ | |||
| Molar mass | 81.09 g/mol | ||
| Related compounds | |||
Related compounds
|
thiopyrylium, selenopyrylium, telluropyrylium | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Pyrylium is a cation (positive ion) with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
Salts[edit]
Pyrylium and its derivatives form stable salts with a variety of anions.[2][3][4][5][6][7]
Derivatives[edit]
Many important cations are formally derived from pyrylium by substitution of various functional groups for some or all the hydrogens in the ring. The 2,4,6-triphenylpyrylium, referred to as the Katritzky salt, (after Alan R. Katritzky) is an important example used in many modern examples of metal catalyzed cross-couplings.[8]
Chemical properties[edit]
Like other oxonium ions, pyrylium is unstable in neutral water. However, pyrylium is much less reactive than ordinary oxonium ions because of aromatic stabilization. The 2,4,6-triphenyl salt is commonly reacted with aliphatic amines at the 1 position, forming pyridinium salts and activating them towards oxidative addition by metal complexes, most notably ones with nickel.[9] Pyrylium cations also react with nucleophiles in the 2, 4, and 6 positions, which can induce a variety of reactions. The high electronegativity of the oxygen results in strong single perturbation by one heteroatom in the six-membered ring.
Synthesis[edit]
Pyrylium salts are easily produced from simple starting materials through a condensation reaction.
Pyrylium salts with aromatic substituents, such 2,4,6-triphenylpyrylium tetrafluoroborate, can be obtained from two moles of acetophenone and one mole of benzaldehyde in the presence of tetrafluoroboric acid and an oxidizing agent (Dilthey synthesis). For pyrylium salts with alkyl substituents, such as 2,4,6-trimethylpyrylium salts, the best method uses the Balaban-Nenitzescu-Praill synthesis from tertiary butanol and acetic anhydride in the presence of tetrafluoroboric, perchloric, or trifluoromethanesulfonic acids.[10][11] 2,4,6-Triphenylpyrylium salts are converted by bases into a stable 1,5-enedione (pseudobase), but 2,4,6-trimethylpyrylium salts on treatment with hot alkali hydroxides afford an unstable pseudobase that undergoes an intramolecular condensation yielding 3,5-dimethylphenol. In warm deuterium oxide, 2,4,6-trimethylpyrylium salts undergo isotopic exchange of 4-methyl hydrogens faster than for the 2- and 6-methyl groups, allowing the synthesis of regioselectively deuterated compounds.
Derivatives[edit]
The reactivity of pyrylium salts toward nucleophiles makes them useful materials for producing other compounds with stronger aromatic character. Pyrylium salts afford pyridines with ammonia,[12] pyridinium salts with primary amines, pyridine-N-oxides with hydroxylamine, phosphabenzenes with phosphine derivatives, thiopyrylium salts with hydrogen sulfide, and benzene derivatives with acetonitrile or nitromethane.
Pyrones[edit]
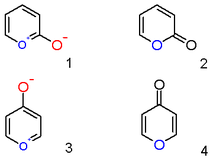
A pyrylium cation with a hydroxyl anion substituent in the 2-position is not the zwitterionic aromatic compound (1), but the neutral unsaturated lactone 2-pyrone or pyran-2-one (2). Important representatives of this class are the coumarins. Likewise a 4-hydroxyl pyrylium compound is a γ-pyrone or pyran-4-one (4), to which group belong compounds such as maltol.
Chemical properties[edit]

2-Pyrones are known to react with alkynes in a Diels–Alder reaction to form arene compounds with expulsion of carbon dioxide, for example:[13]
Polycyclic pyrylium ions[edit]
Chromenylium ion[edit]
One bicyclic pyrylium ion is called benzopyrylium ion (IUPAC: chromenylium ion) (formula: C9H7O+, molar mass: 131.15 g/mol, exact mass: 131.04968983). It can be seen as a charged derivative of 2H-1-benzopyran (IUPAC: 2H-chromene, C9H8O), or a (charged) substituted heterocyclic derivative of naphthalene (C10H8).
Flavylium ion[edit]
In biology, the 2-phenylbenzopyrylium (2-phenylchromenylium) ion is referred to as flavylium. A class of flavylium-derived compounds are anthocyanidins and anthocyanins, pigments that are responsible for the colors of many flowers.[citation needed]
Naphthoxanthenium cation[edit]
Higher polycyclic derivatives of pyrylium also exist. One good example is naphthoxanthenium. This dye is highly stable, aromatic, and planar. It absorbs in the UV and blue region and presents exceptional photophysical properties. It can be synthesized by chemical or photochemical reactions.[14]
-
Benzopyrylium chloride (chromenylium chloride), a salt with chloride as the counterion
-
Flavylium cation
-
Naphthoxanthenium cation
See also[edit]
- 6-membered aromatic rings with one carbon replaced by another group: borabenzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, stibabenzene, bismabenzene, pyrylium, thiopyrylium, selenopyrylium, telluropyrylium
- Pyran, C5H6O (pyrones lacking the ketone group)
References[edit]
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1097. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Gilchrist, T. L. (1997). Heterocyclic Chemistry. ISBN 0-582-27843-0.
- ^ Balaban, A. T.; Schroth, W.; Fischer, G. (1969). Katritzky, A. R.; Boulton, A. J. (eds.). Pyrylium Salts. I. Synthesis. Advances in Heterocyclic Chemistry. Vol. 10. New York: Academic Press. pp. 241–326. doi:10.1016/S0065-2725(08)60499-7.
- ^ Balaban, A. T.; Dinculescu, A.; Dorofeenko, G. N.; Fischer, G. W.; Koblik, A. V.; Mezheritskii, V. V.; Schroth, W. (1982). Katritzky, A. R. (ed.). Pyrylium Salts. Syntheses, Reactions and Physical Properties. Advances in Heterocyclic Chemistry: Supplement. Vol. 2. New York: Academic Press. ISBN 978-0-12-020652-0.
- ^ Balaban, A. T. (1979). "The Pyrylium Cation as a Synthon in Organic Chemistry". In Mitra, R. B.; Ayyangar, N. R.; Gogte, V. N.; Acheson, R. M.; Cromwell, N. (eds.). New Trends in Heterocyclic Chemistry. Studies in Organic Chemistry. Vol. 3. Amsterdam: Elsevier. pp. 79–111. ISBN 978-0-444-41737-4.
- ^ Balaban, A. T. (1987). "Pyrylium Salts as Useful Synthons". In Chizov, O. (ed.). Organic Synthesis: Modern Trends. Oxford: Blackwell. pp. 263–274. ISBN 0-632-02014-8.
- ^ Balaban, T. S.; Balaban, A. T. (2003). "Pyrylium Salts". Hetarenes and Related Ring Systems, Six-membered Hetarenes with one Chalcogen. Science of Synthesis; Houben-Weyl Methods of Molecular Transformations. Vol. 14. Stuttgart: Georg Thieme Verlag. pp. 11–200. ISBN 978-3-13-118641-6.
- ^ Balaban, A. T.; Wray, V. (1977). "13C n.m.r. spectra of some pyrylium salts and related compounds". Organic Magnetic Resonance. 9 (1): 16–22. doi:10.1002/mrc.1270090105.
- ^ Pang, Yue; Moser, Daniel; Cornella, Josep (2020). "Pyrylium Salts: Selective Reagents for the Activation of Primary Amino Groups in Organic Synthesis". Synthesis. 52 (4): 489–503. doi:10.1055/s-0039-1690703. S2CID 208705148.
- ^ Balaban, A. T.; Boulton, A. J. (1973). "2,4,6-Trimethyl-Pyrylium Tetrafluoroborate" (PDF). Organic Syntheses; Collected Volumes, vol. 5, pp. 1112–1113.
- ^ Balaban, A. T.; Boulton, A. J. (1973). "2,4,6-Trimethyl-Pyrylium Trifluoromethanesulfonate" (PDF). Organic Syntheses; Collected Volumes, vol. 5, pp. 1114–1116.
- ^ Anderson, A. G.; Stang, P. J. (1981). "2,6-Di-tert-Butyl-4-Methylpyridine" (PDF). Organic Syntheses. 60: 34; Collected Volumes, vol. 7, p. 144.
- ^ Delaney, P. M.; Moore, J. E.; Harrity, J. P. A. (2006). "An Alkynylboronic Ester Cycloaddition Route to Functionalised Aromatic Boronic Esters". Chemical Communications. 2006 (31): 3323–3325. doi:10.1039/b607322k. PMID 16883424.
- ^ Bucher, G.; Bresolí-Obach, R.; Brosa, C.; Flors, C.; Luis, J. L.; Grillo, T. A.; Nonell, S. (2014). "β-Phenyl quenching of 9-phenylphenalenones: a novel photocyclisation reaction with biological implications". Physical Chemistry Chemical Physics. 16 (35): 18813–18820. Bibcode:2014PCCP...1618813B. doi:10.1039/C4CP02783C. PMID 25079707.