α-ketoglutarate dehydrogenase E1
| α-ketoglutarate dehydrogenase E1 | ||
|---|---|---|

|
||
| Ribbon / surface model of the KDH-E1 dimer from Escherichia coli with thiamine analog as a calotte, according to PDB 2GDJ | ||
| Properties of human protein | ||
| Mass / length primary structure | 983 amino acids | |
| Cofactor | TPP | |
| Identifier | ||
| Gene name | OGDH | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.2.4.2 , oxidoreductases | |
| Response type | Decarboxylation, succinylation | |
| Substrate | 2-ketoglutarate + lipoyllysine DLST | |
| Products | Succinyl lipoyl lysine DLST + CO 2 | |
| Occurrence | ||
| Parent taxon | Bacteria , eukaryotes | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 4967 | 18293 |
| Ensemble | ENSG00000105953 | ENSMUSG00000020456 |
| UniProt | Q02218 | Q60597 |
| Refseq (mRNA) | NM_001003941 | NM_001252282 |
| Refseq (protein) | NP_001003941 | NP_001239211 |
| Gene locus | Chr 7: 44.61 - 44.71 Mb | Chr 11: 6.29 - 6.36 Mb |
| PubMed search | 4967 |
18293
|
α-Ketoglutarate dehydrogenase E1 is the enzyme in bacteria and eukaryotes which, as an E1 subunit of the α-ketoglutarate dehydrogenase complex, catalyzes the decarboxylation of α-ketoglutarate . This reaction is part of the citric acid cycle . In addition, α-ketoadipate can be decarboxylated, which is necessary for the breakdown of the amino acid lysine . The protein complex is located in the mitochondria . Rare mutations in OGDH - gene can and E1 deficiency of the rare Ketoglutarazidurie lead.
Catalyzed reaction
The decarboxylation of ketoglutarate place on thiamine as catalytic center instead of forming an atomic bond with ketoglutarate, so succinyl thiamine - pyrophosphate with elimination of CO 2 is produced.
![]() + + CO 2 (R = OOC-CH 2 -CH 2 -)
+ + CO 2 (R = OOC-CH 2 -CH 2 -)


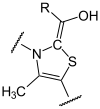
This succinyl residue (syn. Succinic acid ) of the TPP is taken over by the α-lipoic acid ( oxidation ). It is covalently bound to the Lipoat DSLT subunit. S-succinyl hydrolip (oat / onamide) is formed.
The reaction is thus equivalent to the decarboxylation of pyruvate by pyruvate dehydrogenase E1 .
literature
- Bunik V, Kaehne T, Degtyarev D, Shcherbakova T, Reiser G: Novel isoenzyme of 2-oxoglutarate dehydrogenase is identified in brain, but not in heart . In: FEBS J . . 275, No. 20, October 2008, pp. 4990-5006. doi : 10.1111 / j.1742-4658.2008.06632.x . PMID 18783430 .
Individual evidence
- ↑ UniProt Q02218
- ↑ Orphanet: Oxoglutaric aciduria


