γ-decalactone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
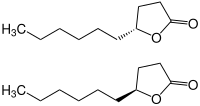
|
||||||||||||||||
| ( R ) -isomer (top) and ( S ) -isomer (bottom) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | γ-decalactone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 18 O 2 | |||||||||||||||
| Brief description |
colorless to slightly yellowish liquid with a fruity odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 170.25 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.952 g cm −3 |
|||||||||||||||
| boiling point |
281 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4489 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
γ-decalactone is the lactone of γ-hydroxydecanoic acid and is used as a flavoring substance in various foods . A distinction must be made between two chiral configurations (see Cahn-Ingold-Prelog Convention ):
- ( R ) - (+) - γ-decalactone has a peach-like , fruity aroma
- ( S ) - (-) - γ-decalactone has a coconut-like , fatty aroma
Occurrence
( R ) - (+) - γ-decalactone occurs mostly in nature, the enantiomeric excess often being over 80%. This is decisive for the aroma of apricots (apricots, Prunus armeniaca ) and - together with other lactones - is also decisive for the aroma of peaches ( Prunus persica ). But also in other fruits, e.g. B. in pineapple , strawberries , passion fruit and mango , ( R ) - (+) - γ-decalactone is contained as a flavoring substance.
The flavor of Camembert contains γ-decalactone. However, the δ-decalactone is more important for the taste of cheese .
Manufacturing
( R ) -γ-decalactone is obtained with the help of microorganisms (yeasts and fungi such as Candida, Monilia, Sporobolomyces, etc.) fermentatively via the intermediate stage ricinoleic acid from castor oil and added to foods . Rare γ-decalactone is made of ethyl decanoate (often isolated from coconut oil) by means of Mucor circinelloides generated. Γ-decalactone can be obtained synthetically from 3-bromodecanoic acid after heating with sodium carbonate .
Individual evidence
- ↑ a b c d Entry on decanolactones. In: Römpp Online . Georg Thieme Verlag, accessed on August 31, 2015.
- ↑ a b c d data sheet γ-Decalactone, ≥98% from Sigma-Aldrich , accessed on February 3, 2018 ( PDF ).
- ↑ Laurette Tavel, Isabelle Andriot, Celine Moreau, Elisabeth Guichard: Interactions between β-Lactoglobulin and Aroma Compounds: Different Binding Behaviors as a Function of Ligand Structure . October 18, 2008, doi : 10.1021 / jf801841u .
- ↑ Data sheet page no longer available , search in web archives: γ-decalactone (PDF; 51 kB) at Lansdowne Aromatics, accessed on April 2, 2012.
- ^ MS Kharasch, PS Skell, Paul Fisher: Reactions of Atoms and Free Radicals in Solution. XII. The Addition of Bromo Esters to Olefins . In: Journal of the American Chemical Society . tape 70 , no. 3 , March 19, 1948, ISSN 0002-7863 , p. 1055-1059 , doi : 10.1021 / ja01183a053 .
- ↑ W. Ternes: Scientific basics of food preparation. 3. Edition. Behr's Verlag, Hamburg 2008, p. 316 ( limited preview in Google book search).
- ^ A b Hans-Dieter Belitz, Werner Grosch, Peter Schieberle: Food Chemistry . 4th edition. Springer Science & Business Media, Berlin Heidelberg 2009, ISBN 978-3-540-69933-0 , 5.2 Aroma Analysis, p. 355 , doi : 10.1007 / 978-3-540-69934-7 (English, limited preview in the Google book search [accessed on 23 August 2016] enantiomeric excess> 80%).
- ↑ a b Kathrin Verena Eisinger: Flavors in selected everyday foods . Diploma thesis, University of Vienna, Faculty of Life Sciences, Supervisor: Dorota Majchrzak. Ed .: University of Vienna. Vienna December 2008, 3.1.2.1. Apricot, 3.3.3. Apricot juice, 3.3.4. Peach juice, 3.4.4.1.4. Soft cheese, S. 52–53, 89–91, 115–116, 127, 129 ( online [PDF; 1.5 MB ; accessed on August 23, 2016]).
- ^ Leo ML Nollet, Fidel Toldra: Handbook of Dairy Foods Analysis . CRC Press, Taylor & Francis, Boca Raton 2009, ISBN 978-1-4200-4632-8 , 12.3.3 Lipolysis and Catabolism of Fatty Acids, pp. 288 (English, limited preview in Google Book Search [accessed August 23, 2016]).
- ↑ U.-J. Salzer, F. Siewek (Ed.): Handbook of Aromas and Spices. Behr's Verlag, Hamburg 1999, pp. 20–21 ( limited preview in the Google book search).
