δ-decalactone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structure without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | δ-decalactone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 18 O 2 | ||||||||||||||||||
| Brief description |
colorless, viscous liquid with a milky-creamy aroma |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 170.25 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.954 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
−27 ° C |
||||||||||||||||||
| boiling point |
117–120 ° C (0.03 h Pa ) |
||||||||||||||||||
| solubility |
sparingly soluble in water (4 g l −1 at 28 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
δ-decalactone is an organic chemical compound and belongs to the class of lactones . It occurs as a flavoring in food - especially dairy products - and is a key flavoring in butter .
Isomers
δ-decalactone occurs in two enantiomeric forms [( R ) -δ-decalactone and ( S ) -δ-decalactone]. The 1: 1 mixture of the two compounds, the racemate , is referred to as ( RS ) -δ-decalactone.
| Isomers of δ-decalactone | ||
| Surname | ( S ) -δ-decalactone | ( R ) -δ-decalactone |
| Structural formula |

|

|
| CAS number | 59285-67-5 | 2825-91-4 |
| 705-86-2 (racemate) | ||
| EC number | - | - |
| 211-889-1 (racemate) | ||
| ECHA info card | - | - |
| 100.010.810 (racemate) | ||
| PubChem | 6566010 | 1714996 |
| 12810 (racemate) | ||
| Wikidata | Q27273761 | Q27256617 |
| Q27159530 (racemate) | ||
Occurrence
δ-decalactone occurs as a flavoring substance in various foods. It is one of the most important components of butter flavor and also contributes to the flavor of other dairy products such as long-life milk and various cheeses . It is also found in various fruits such as peach , apricot , coconut or raspberry . It creates an off-flavor in meat.
Most sources other than raspberries are largely in the ( R ) form.
Extraction and presentation
In nature, δ-decalactone is produced in several steps from linoleic acid . The carbon chain is shortened by β-oxidation . The direct precursor of δ-decalactone is 5-hydroxydecanoic acid, which is esterified within the molecular structure with ring closure . Industrially, for example, δ-decalactone can be produced by fermentation from massoia oil, an oil from the bark of the tropical tree Cryptocarya massoia .
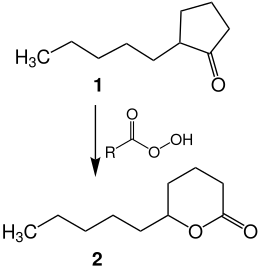
δ-decalactone ( 2 ) will also be produced synthetically on an industrial scale. It is produced by the oxidation of 2-pentylcyclopentanone ( 1 ) with a peroxycarboxylic acid ( Baeyer-Villiger oxidation ). The configuration of the ketone is retained in the lactone.
properties
Aroma properties
The smell and taste of the two enantiomers do not differ much from one another. As with all flavorings, the perception is different. δ-decalactone is perceived as strong, sweet, creamy, coconut-like, fruity and peach-like in taste and smell. Elsewhere, the aroma is described as milky and creamy.
The odor threshold of δ-decalactone is 100 μg / kg water.
Chemical properties
Of δ-decalactone can be obtained by ring-opening polymerization of the polyester poly-δ-decalactone can be produced. Also, a copolymerization with other monomers is also possible.
use
δ-decalactone is used as a fragrance in perfumes and in butter, cream and cheese flavors.
The polymer poly-δ-decalactone and some copolymers are currently being researched as possible bio-based plastics . These polymers are seen to have potential in the area of drug targeting .
Individual evidence
- ↑ a b c d e f g Entry on decanolactones. In: Römpp Online . Georg Thieme Verlag, accessed on November 26, 2019.
- ↑ a b c d data sheet δ-Decalactone natural, ≥98%, FCC, FG from Sigma-Aldrich , accessed on November 26, 2019 ( PDF ).
- ↑ a b Entry on decan-5-olide in the GESTIS substance database of the IFA , accessed on November 26, 2019 (JavaScript required)
- ↑ a b Hans-Dieter Belitz, Werner Grosch & Peter Schieberle: Textbook of food chemistry . 6th edition. Springer, Berlin 2008, ISBN 978-3-540-73201-3 , 10.3, pp. 557-563 , doi : 10.1007 / 978-3-540-73202-0 .
- ^ A b c Hans-Dieter Belitz, Werner Grosch & Peter Schieberle: Textbook of food chemistry . 6th edition. Springer, Berlin 2008, ISBN 978-3-540-73201-3 , 5.2.3.2, pp. 387-389 , doi : 10.1007 / 978-3-540-73202-0 .
- ↑ a b c d Jens Schrader: Microbial Flavor Production . In: Ralf Günter Berger (Ed.): Flavors and Fragrances - Chemistry, Bioprocessing and Sustainability . Springer, Berlin 2007, ISBN 978-3-540-49338-9 , 23.4.4, pp. 556-557 , doi : 10.1007 / b136889 .
- ↑ Avelino Corma, Sara Iborra, MarÌa Mifsud, Michael Renz & Manuel Susarte: A New Environmentally Benign Catalytic Process for the Asymmetric Synthesis of Lactones: Synthesis of the Flavoring δ-Decalactone Molecule . In: Advanced Synthesis & Catalysis . tape 346 , 2004, pp. 257-262 , doi : 10.1002 / adsc.200303234 .
- ↑ a b c d e f Bettina Muermann & Uwe-Jens Salzer: Aromen-Lexikon . Behr's Verlag, 2015, ISBN 978-3-95468-359-8 , pp. 47 ( limited preview in Google Book search).
- ^ A b Mark T. Martello, Adam Burns & Hillmyer: Bulk Ring-Opening Transesterification Polymerization of the Renewable δ-Decalactone Using an Organocatalyst . In: ACS Macro Letters . tape 1 , 2012, p. 131-135 , doi : 10.1021 / mz200006s .
- ↑ a b Deborah K. Schneiderman, Chad Gilmer, Michael T. Wentzel, Mark T. Martello, Tomohiro Kubo & Jane E. Wissinger: Sustainable Polymers in the Organic Chemistry Laboratory: Synthesis and Characterization of a Renewable Polymer from δ ‑ Decalactone and L ‑Lactide . In: Journal of Chemical Education . tape 91 , 2014, p. 131-135 , doi : 10.1021 / ed400185u .
- ↑ Kuldeep K. Bansal, Deepak Kakde, Laura Purdie, Derek J. Irvine, Steven M. Howdle, Giuseppe Mantovani & Cameron Alexander: New biomaterials from renewable resources - amphiphilic block copolymers from δ-decalactone . In: Polymer Chemistry . tape 6 , 2015, p. 7196-7210 , doi : 10.1039 / c5py01203a .