1-methyl nicotinamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
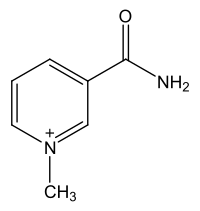
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-methyl nicotinamide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula |
|
|||||||||||||||
| Brief description |
White dust |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | ||||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
207-209 ° C (chloride) |
|||||||||||||||
| solubility |
easily in water (15 mg ml −1 at 20 ° C) (chloride) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1-methylnicotinamide is the methylated amide of nicotinic acid (niacin, vitamin B 3 ). It is a substance produced by the body when nicotinic acid is metabolized in the liver.
Occurrence
The highest natural concentration of 1-methylnicotinamide can be found in the alga Undaria pinnatifida . It is also found in the Judas ear mushroom and green tea .
Extraction and presentation
1-methylnicotinamide is primarily produced in the liver by the nicotinamide N-methyltransferase. The reaction takes place during the metabolism of NAD ( nicotinamide adenine dinucleotide ). 1-Methylnicotinamide can be produced synthetically with methylation reagents and nicotinamide .
use
For a long time, 1-methylnicotinamide was considered a biologically inactive metabolite of nicotinamide . However, various studies show antithrombotic, anti-inflammatory, gastroprotective and vasoprotective properties.
1-methylnicotinamide is an endogenous activator of prostacyclin synthesis and can therefore regulate trombolytic and inflammatory processes in the cardiovascular system. It inhibits platelet-dependent thrombosis through a mechanism involving cyclooxygenase-2 and prostacyclin and increases nitric oxide bioavailability in the endothelium .
Animal experiments with diabetic rats have shown that 1-methylnicotinamide has a positive influence on degenerative changes in the brain and thus cognitive performance can be maintained for longer.
Experiments with the nematode Caenorhabditis elegans showed that the addition of 1-methylnicotinamide can extend their lifespan. This can possibly be attributed to an increased binding of free radicals and the resulting reduced oxidative stress .
1-methylnicotinamide is used in cosmetics, such as hair and skin care products, and as a dietary supplement.
Individual evidence
- ↑ a b c d e data sheet 1-Methylnicotinamide Chloride from Sigma-Aldrich , accessed on January 29, 2020 ( PDF ).
- ↑ External identifiers of or database links to 3-carbamoyl-1-methylpyridinium chloride : CAS number: 1005-24-9, EC number: 463-670-7, ECHA InfoCard: 100.104.777 , PubChem : 70495 , ChemSpider : 63668 , Wikidata : Q27282725 .
- ↑ External identifiers or database links to 3-carbamoyl-1-methylpyridinium iodide: CAS number: 6456-44-6, EC number: 229-263-1 , ECHA InfoCard: 100.026.603 , PubChem : 72660 , ChemSpider : 65521 , Wikidata : Q82970083 .
- ↑ Kosower, EM; Klinedinst, PE: "Additions of pyridinium rings. II. Charge-transfer complexes as intermediates" in J. Am. Chem. Soc. 78 (1956) 3493-3497.
- ↑ a b Taguchi, H .; Sakaguchi, M .; Shimbabayashi, Y .: "Contents of quinolinic acid trigonelline and N-1 methylnicotinamide in various foods and thermal conversion of these compounds into nicotinic acid and nicotinamide" in [Vitamins] 60 (1986) 537-546.
- ↑ Gebicki, J .; Sysa-Jedrzejowska, A .; Adamus, J .; Wozniacka, A .; Rybak, M .; Zielonka, J .: "1-methylnicotinamide: a potent anti-inflammatory agent of vitamin origin" in Pol. J. Pharmacol. 55 (2003) 109-112.
- ↑ Bryniarski, K .; Biedron, R .; Jakubowski, A .; Chlopicki, S .; Marcinkiewicz, J .: "Anti-inflammatory effect of 1-methylnicotinamide in contact hypersensitivity to oxazolone in mice; involvement of prostacyclin" in Eur. J. Pharmacol. 578 (2008) 332-338.
- ↑ a b c Domagala, TB; Szeffler, A .; Dobrucki, LW; Dropinski, J .; Polanski, S .; Leszczynska-Wiloch, M .; Kotula-Horowitz, K .; Wojciechowski, J .; Wojnowski, L .; Szczeklik, A .; Kalinowski, L .: "Nitric oxide production and endothelium-dependent vasorelaxation ameliorated by N1-methylnicotinamide in human blood vessels" in Hypertension 59 (2012) 825-832.
- ↑ Bartus, M .; Lomnicka, M .; Kostogrys, RB; Kazmierczak, P .; Watala, C .; Slominska, EM; Smolenski, RT; Pisulewski, PM; Adamus, J .; Gebicki, J .; Chlopicki, S .: "1-Methylnicotinamide (MNA) prevents endothelial dysfunction in hypertriglyceridemic and diabetic rats" in Pharmacol. Rep. 60 (2008) 127-138.
- ↑ Chlopicki, S .; Swies, J .; Mogielnicki, A .; Buczko, W .; Bartus, M .; Lomnicka, M .; Adamus, J .; Gebicki, J .: "1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2 / prostacyclin pathway" in Br. J. Pharmacol. 152 (2007) 230-239.
- ↑ Kuchmerovska, T .; Shymanskyy, I .; Chlopicki, S .; Klimenko, A.: "1-methylnicotinamide (MNA) in prevention of diabetes-associated brain disorders." in Neurochem. Int. 56 (2010) 221-228.
- ↑ Schmeisser, K .; Mansfeld, J .; Kuhlow, D .; Weimer, S .; Priebe, S .; Savior, I .; Birringer, M .; Groth, M .; Segref, A .; Kanfi, Y .: "Role of Sirtuins in Lifespan Regulation is Linked to Methylation of Nicotinamide" in Nat. Chem. Biol. 9 (2013) 693-700.
- ↑ Entry of 1-methylnicotinamide on startupvalley.de , accessed on January 29, 2020.