2-naphthoic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-naphthoic acid | ||||||||||||||||||
| other names |
β-naphthoic acid |
||||||||||||||||||
| Molecular formula | C 11 H 8 O 2 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 172.18 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.08 g cm −3 |
||||||||||||||||||
| Melting point |
182-186 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2- Naphthoic acid, also known as β-naphthoic acid, is a naphthalene derivative. These occur in coal tar. They belong to the substance class of condensed aromatic hydrocarbons . These absorb light in the UV range and show fluorescence . 2-naphthoic acid is isomeric to 1-naphthoic acid .
presentation
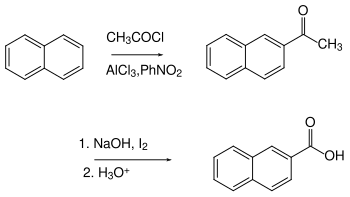
2-Naphthoic acid can be synthesized from naphthoquinone by reduction with KOH .
Another common method is the synthesis of 2-napthoic acid from naphthalene: The first step is the preparation of a ketone by Friedel-Crafts acylation . A subsequent haloform reaction is a synthetically useful method for the oxidation of the methyl ketone to the carboxylic acid.
On the other hand, 2-naphthoic acid cannot be synthesized by oxidation of 2-methylnaphthalene, since the substituted ring is more easily oxidizable than the side chain. In the case of the electrophilic substitution of naphthalene, the α-position is normally preferred.
properties
It is a colorless solid.
- 1 H-NMR: 10.05 ppm
- 13 C-NMR: 127.5; 127.8; 128.4 ppm
- Mass spectrometry: m / z 127, 155, 172.
use
They are used as a raw material in the manufacture of pigments, dyes and pharmaceuticals.
Acid strength (p K S values) of the singlet and triplet states
From the pH-dependent absorption and fluorescence of 2-naphthoic acid, after considering the thermodynamic equilibrium of the undissociated acid and the acid anion, the p K S value in the respective energy state can be calculated:
| p K S0 | p K S1 | p K T1 | p K T1 |
| 4.2 | 10-12 | 4.0 | 4.2 |
The p K S values have been determined by measuring fluorescence and phosphorescence. This shows that the S 1 state is more basic than the ground state S 0 .
Degradation of pollutants
The degradation of naphthoic acid takes place through reduction with the help of microorganisms under anaerobic conditions:
literature
- A. Streitwieser, CH Heathcock: Organic Chemistry. Wiley-VCH, Weinheim et al. 1986, ISBN 3-527-25810-8 .
- K. Peter C. Vollhardt: Organic Chemistry. VCH, Weinheim et al. 1990, ISBN 3-527-26912-6 .
Individual evidence
- ↑ a b c d e f g h data sheet 2-Naphthoic acid, 98 +% from AlfaAesar, accessed on November 27, 2017 ( PDF )(JavaScript required) .
- ^ S. Gabriel, Ernst Leupold: Transformations of the Aethindiphtalids. II. In: Reports of the German Chemical Society. 31, 1898, pp. 1272-1286, doi : 10.1002 / cber.18980310204 .
- ↑ H. Meislich, H. Nechamkin, J. Sharefkin: Organic Chemistry. Schaum, Mc Graw Hill Book Company 1980, ISBN 0-07-092028-2 , p. 394.
- ↑ Jürgen Kiefer, J. Bensel: Ultraviolet rays. W. de Gruyter, 1977, ISBN 3-11-082276-8 , p. 361.
- ↑ Thomas Held: In Situ Procedure for Soil and Groundwater Remediation. Wiley, ISBN 978-3-527-68183-9 .