2,5-dimethoxy-4-iodophenethylamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
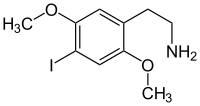
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,5-dimethoxy-4-iodophenethylamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 14 INO 2 | ||||||||||||||||||
| Brief description |
white crystals (hydrochloride) |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 307.13 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
246 ° C (hydrochloride) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,5-Dimethoxy-4-iodophenethylamine (abbreviated 2C-I ) is a psychedelic , which, due to its structure, belongs to the substance groups of phenol ethers , phenethylamines , as well as to the substance group of 2Cs .
history
The American chemist Alexander Shulgin synthesized 2C-I for the first time and mentions synthesis, dose and effect in his book PiHKAL .
Pharmacodynamics
The effect is described as stimulating , entactogenic / empathogenic and entheogenic , among other things . This is attributed, among other things, to the mode of action of the 2C-Is as a potent agonist of the serotonin receptors 5-HT 2A / 2C . 2C-I acts (among other things) as a serotonin-noradrenaline reuptake inhibitor (IC 50 5-HT: 79 ± 19 μM NE: 37 ± 12 μM).
Pharmacokinetics
Shulgin specifies the orally effective dose in the range from 14 to 22 mg, the duration of action as 6-10 hours.
Legal status
2C-I is a non-marketable narcotic drug in the Federal Republic of Germany due to its listing in Appendix I BtMG . Handling without permission is generally a criminal offense.
See also
- 25I-NBOMe , a 2C-I derivative
literature
- Alexander Shulgin, Ann Shulgin: PIHKAL - A Chemical Love Story Transform Press, ISBN 0-9630096-0-5 .
Web links
Individual evidence
- ↑ a b c d PiHKAL # 33 2C-I
- ↑ There is not yet a harmonized classification for this substance . A label of [No public or meaningful name is available] in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 16, 2020, is derived from a self-classification by the distributor .
- ↑ CA Villalobos, P. Bull, P. Sáez, BK Cassels, JP Huidobro-Toro: 4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT 2A receptor antagonists in Xenopus laevis oocytes , in: Br. J. Pharmacol. , 2004 , 141 (7) , 1167-1174; PMC 1574890 (free full text).
- ↑ Pablo R. Moya, Kelly A. Berg, Manuel A. Gutiérrez-Hernandez, Patricio Sáez-Briones, Miguel Reyes-Parada, Bruce K. Cassels, William P. Clarke: "Functional Selectivity of Hallucinogenic Phenethylamine and Phenylisopropylamine Derivatives in Human 5 -Hydroxytryptamine (5-HT) 2A and 5-HT 2C Receptors ", in: Journal of Pharmacology and Experimental Therapeutics , 2007 , 321 (3) , 1054-1061; doi : 10.1124 / jpet.106.117507 .
- ↑ F. Nagai, R. Nonaka, K. Satoh Hisashi Kamimura: The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. In: European journal of pharmacology. Volume 559, Number 2-3, March 2007, pp. 132-137, doi : 10.1016 / j.ejphar.2006.11.075 . PMID 17223101 .

