2C (substance group)
2C ( 2C-x ) is the group name of the psychedelic phenethylamines , which have a methoxy group in the second and fifth positions of the phenethylamine stem structure . Most of these substances have a lipophilic substituent in the fourth position (para-substituted) , which influences the potency and the degradation of the substance. Many of these 2C substances were first synthesized by Alexander Shulgin in the 1970 / 80s and described in his book PIHKAL ( Phenethylamines I Have Known And Loved ). Shulgin is also the namesake of this group of substances, which names the two carbon atoms ( carbon ) between the benzene ring and the amino group . Some of these 2C substances are used as ligands in the elucidation of structure-activity relationships in the 5-HT 2A / 2C receptors .
Table overview
| Surname | R 3 | R 4 | Structural formula |
|---|---|---|---|
| 2C-B | - H. | - Br |

|
| 2C-C | -H | - Cl |
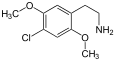
|
| 2C-D | -H | - CH 3 |

|
| 2C-E | -H | - CH 2 CH 3 |
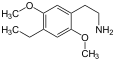
|
| 2C-F | -H | - F |
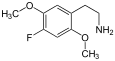
|
| 2C-G | -CH 3 | -CH 3 |
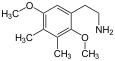
|
| 2C-G-3 | - (CH 2 ) 3 - |

|
|
| 2C-G-4 | - (CH 2 ) 4 - |

|
|
| 2C-GN | - (CH) 4 - |

|
|
| 2C-H | -H | -H |

|
| 2C-I | -H | - I. |

|
| 2C-N | -H | - NO 2 |

|
| 2C-O | -H | - OCH 3 |

|
| 2C-O-4 | -H | - OCH (CH 3 ) 2 |

|
| 2C-P | -H | - CH 2 CH 2 CH 3 |

|
| 2C-SE | -H | - Se CH 3 |

|
| 2C-T | -H | - SCH 3 |

|
| 2C-T-2 | -H | - SCH 2 CH 3 |
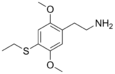
|
| 2C-T-4 | -H | - SCH (CH 3 ) 2 |
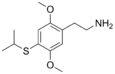
|
| 2C-T-7 | -H | -S (CH 2 ) 2 CH 3 |
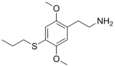
|
| 2C-T-8 | -H | - SCH 2 CH (CH 2 ) 2 |
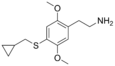
|
| 2C-T-9 | -H | -SC (CH 3 ) 3 |
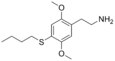
|
| 2C-T-13 | -H | -S (CH 2 ) 2 OCH 3 |

|
| 2C-T-15 | -H | - SCH (CH 2 ) 2 |

|
| 2C-T-17 | -H | - SCH (CH 3 ) CH 2 CH 3 |
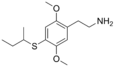
|
| 2C-T-21 | -H | -S (CH 2 ) 2 F |

|
| 2C-TFM | -H | - CF 3 |

|
| 2C-YN | -H | - C≡CH |
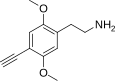
|
| 2C-V | -H | - CH = CH 2 | - |
| 2C-CN | -H | - C≡N | - |
| 2C-CA | -H | - COOH | - |
| 2C-BI-1 | -H | - C 6 H 5 | - |
| 2C-IP | -H | - C 3 H 7 | - |
| 2C-HM | -H | - CH 2 -OH | - |
| 2C-NH | -H | - NH 2 | - |
literature
- Trachsel D., Lehmann D., Enzensperger Ch .: Phenethylamine From Structure to Function , Nightshade Science Edition, Solothurn, Switzerland, 2013 ISBN 978-3-03788-700-4
- Alexander T. Shulgin, Tania Manning, Paul F. Daley: The Shulgin Index: Psychedelic Phenethylamines and Related Compounds, Volume One . Transform Press, Berkeley 2011, ISBN 0-9630096-3-X .
Web links
Individual evidence
- ↑ Daniel Trachsel: Synthesis of new (phenylalkyl) amines for the investigation of structure-activity relationships. Communication 2. In: Helvetica Chimica Acta . 86, 2003, pp. 2610-2619, doi : 10.1002 / hlca.200390210 .
- ↑ Antoni R. Blaazer, Pieter Smid, Chris G. Kruse: Structure-Activity Relationships of Phenylalkylamines as agonist ligand for 5-HT Receptors. In: ChemMedChem . 3, 2008, pp. 1299-1309, doi : 10.1002 / cmdc.200800133 .
- ↑ Ahmet Altun, Kurtulus Golcuk, Mustafa Kumru, Abraham F. Jalbout: Electron-conformational study for the structure-hallucinogenic activity relationships of phenylalkylamines. In: Bioorganic & Medicinal Chemistry . 11, 2003, pp. 3861-3868, doi : 10.1016 / S0968-0896 (03) 00437-1 .
- ^ Thomas S. Ray, Olivier Jacques Manzoni: Psychedelics and the Human Receptorome. In: PLoS ONE . 5, 2010, p. E9019, doi : 10.1371 / journal.pone.0009019 .
