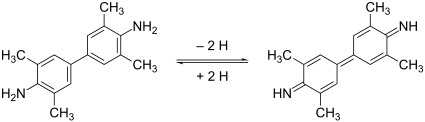3,3 ', 5,5'-tetramethylbenzidine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3,3 ', 5,5'-tetramethylbenzidine | ||||||||||||||||||
| other names |
TMB |
||||||||||||||||||
| Molecular formula | C 16 H 20 N 2 | ||||||||||||||||||
| Brief description |
white, faint-smelling crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 240.34 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.45 g cm −3 ( bulk density ) |
||||||||||||||||||
| Melting point |
168-171 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
3,3 ', 5,5'-Tetramethylbenzidine is a chemical compound from the class of benzidines and is used as a chromogen in immunohistochemistry .
After activation by peroxidase , TMB is blue with an absorption maximum at 650 nm. When sulfuric acid is added, TMB turns yellow at 450 nm. TMB is photosensitive.
use
TMB is used in biochemistry due to its color properties in the course of immunostaining , e.g. B. used in ELISA . Together with hydrogen peroxide and a phosphate-citrate buffer , TMB is used as a reaction starter. The fixation solution (sulfuric acid) brings about the color change after the samples have turned blue thanks to the TMB. If the reaction has stopped, the ELISA is evaluated with an ELISA reader ( photometer ).
Individual evidence
- ↑ a b c d e f data sheet 3,3 ′, 5,5′-tetramethylbenzidine, ≥99% from Sigma-Aldrich , accessed on June 12, 2013 ( PDF ).
- ↑ TL Martin, EJ Mufson, MM Mesulam: The light side of horseradish peroxidase histochemistry . In: J Histochem Cytochem . 32, No. 7, 1984, p. 793. PMID 6736628 .