3-hydroxytetrahydrofuran
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
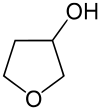
|
|||||||||||||||||||
| Simplified structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3-hydroxytetrahydrofuran | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 88.11 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.09 g cm −3 (25 ° C) |
||||||||||||||||||
| boiling point |
181 ° C |
||||||||||||||||||
| Refractive index |
1.45 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
3-Hydroxytetrahydrofuran is a chemical compound belonging to the furans group .
synthesis
3-Hydroxytetrahydrofuran was produced by M. Pariselle in 1910 by the cyclization and hydrolysis of 3,4-dibromo-1-methoxybutane .
Stereochemistry
3-Hydroxytetrahydrofuran is chiral . Enantiomerically pure 3-hydroxytetrahydrofuran can be synthesized with high purity from ( S ) - or ( R ) - 1,2,4-butanetriol . Thus, the ( S ) -1,2,4-butanetriol can be cyclized to ( S ) -3-hydroxytetrahydrofuran in the presence of catalytic amounts of p -toluenesulfonic acid at temperatures from 180 to 220.degree .
properties
3-Hydroxytetrahydrofuran is a colorless liquid.
use
3-Hydroxytetrahydrofuran can be used as an intermediate in the manufacture of other chemical compounds (such as the drugs amprenavir and fosamprenavir ).
Individual evidence
- ↑ a b c d e f Entry on 3-hydroxytetrahydrofuran at TCI Europe, accessed on January 4, 2017.
- ↑ a b c data sheet 3-Hydroxytetrahydrofuran from Sigma-Aldrich , accessed on January 4, 2017 ( PDF ).
- ^ M. Pariselle: Sur quelques dérivés du butanetriol-1.2.4 . In: Comptes rendus de l'Académie des sciences . No. 149, 1910, pp. 295-298. PDF .
- ↑ Vishnu K. Tandon, Albert M. Van Leusen, Hans Wynberg: Synthesis of enantiomerically pure (S) - (+) - 3-hydroxytetrahydrofuran, and its (R) -enantiomer, from malic or tartaric acid . In: The Journal of Organic Chemistry . tape 48 , no. 16 , August 1, 1983, pp. 2767-2769 , doi : 10.1021 / jo00164a027 .
- ↑ Venkata Mandava, et al .: Preparation of fosamprenavir calcium . US Pat. Appl., 20110224443 (2011).
