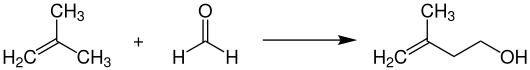Isoprenol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isoprenol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 10 O | |||||||||||||||
| Brief description |
colorless liquid with an alcohol-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 86.13 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.86 g cm −3 |
|||||||||||||||
| Melting point |
−20 ° C |
|||||||||||||||
| boiling point |
129 ° C |
|||||||||||||||
| Vapor pressure |
26.7 mbar (20 ° C) |
|||||||||||||||
| solubility |
soluble in water (90 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.433 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Isoprenol ( 3-methyl-3-buten-1-ol ) is a chemical compound from the group of alkenols .
Extraction and presentation
For the industrial synthesis of isoprenol, isobutene is reacted with liquid formaldehyde at temperatures of 200-300 ° C. and pressures of 250 bar in a reactor without a catalyst.
The product is worked up and purified by multi-stage distillation under pressure in rectification columns .
properties
3-Methyl-3-buten-1-ol is a flammable, volatile, colorless liquid with an alcoholic odor, which is soluble in water.
The OH group is in the homoallyl position in relation to the double bond .
use
3-Methyl-3-buten-1-ol is a building block in the biogenesis of isoprenoids . Senecioaldehyde can be obtained from isoprenol by air oxidation on a silver catalyst .
safety instructions
The vapors of 3-methyl-3-buten-1-ol can form an explosive mixture with air ( flash point 42 ° C, ignition temperature 345 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on 3-methyl-3-buten-1-ol in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Data sheet 3-methyl-3-buten-1-ol, ≥ 97% from Sigma-Aldrich , accessed on December 6, 2011 ( PDF ).
- ↑ a b H. Pommer, A. Nürrenbach: INDUSTRIAL SYNTHESIS OF TERPENE COMPOUNDS. In: Organic Synthesis . 1975, pp. 527-551, doi : 10.1016 / B978-0-408-70725-1.50016-1 . ISBN 978-0-408-70725-1 (book chapter)
- ↑ Hans Martin Weitz, Eckhard Loser: Isoprene . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., June 15, 2000, p. 86, doi : 10.1002 / 14356007.a14_627 .
- ↑ K. Hüsnü, Can Başer, Gerhard Buchbauer: Handbook of Essential Oils: Science, Technology, and Applications , 2nd edition, Boca Raton, 2016, ISBN 978-1-4665-9046-5 , p. 192 ( limited preview in Google Book Search).
- ↑ Patent application WO2008037693 : Continuous process for the production of citral. Registered on September 26, 2006 , published on April 3, 2008 , applicant: BASF SE, inventor: Günter Wegner, Gerd Kaibel, Jörg Therre, Werner Aquila and Hartwig Fuchs.