3-methylpentane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
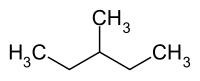
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-methylpentane | |||||||||||||||
| other names |
1,2,3-trimethylpropane |
|||||||||||||||
| Molecular formula | C 6 H 14 | |||||||||||||||
| Brief description |
colorless liquid with a phenolic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 86.18 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.66 g cm −3 |
|||||||||||||||
| Melting point |
−118 ° C |
|||||||||||||||
| boiling point |
63 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
practically insoluble in water (0.018 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.376 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-methylpentane (also diethylmethylmethane) is one of the five constitutional isomers of hexane .
Presentation and extraction
3-methylpentane occurs in petroleum . A technical synthesis is achieved by reacting n-butane with ethylene at 400 ° C. and 200 bar in the presence of homogeneous catalysts such as phosphorus trichloride .
properties
Physical Properties
3-methylpentane is a highly flammable, volatile, colorless liquid with a phenol- like odor. The compound boils at 63 ° C. under normal pressure. According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.97377, B = 1152.368 and C = −46.021 in the temperature range from 289 K to 337 K.
The most important thermodynamic properties are listed in the following table:
| property | Type | Value [unit] |
|---|---|---|
| Standard enthalpy of formation | Δ f H 0 gas | −171.6 kJ mol −1 |
| Standard entropy | S 0 liquid S 0 g |
292.5 J mol −1 K −1 as a liquid 382.88 J mol −1 K −1 as a gas |
| Enthalpy of combustion | Δ c H 0 liquid | −4159.98 kJ mol −1 |
| Heat capacity | c p | 191.16 J mol −1 K −1 (25 ° C) as a liquid |
| Enthalpy of fusion | Δ f H 0 | 5.3032 kJ mol −1 at the melting point |
| Entropy of fusion | Δ f S 0 | 48.101 kJ mol −1 at the melting point |
| Enthalpy of evaporation | Δ V H 0 | 28.08 kJ mol −1 at the normal pressure boiling point of 30.47 kJ mol −1 at 25 ° C |
| Critical temperature | T C | 231 ° C |
| Critical pressure | P C | 31.1 bar |
| Critical volume | V C | 0.368 l mol −1 |
| Critical density | ρ C | 2.72 mol·l −1 |
The temperature dependence of the enthalpy of vaporization can be calculated according to the equation Δ V H 0 = A exp (−β T r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / T c ) reduced temperature) with A = 45.24 kJ / mol, β = 0.2703 and T c = 504.4 K in the temperature range between 298 K and 353 K.
Vapor pressure function of 3-methylpentane
Temperature dependence of the heat of vaporization of 3-methylpentane
Safety-related parameters
3-methylpentane forms highly flammable vapor-air mixtures. The compound has a flash point of less than −20 ° C. The explosion range is between 1.2 vol.% (40 g / m 3 ) as the lower explosion limit (LEL) and 7.0 vol.% (250 g / m 3 ) as the upper explosion limit (UEL). It is assigned to explosion group IIB). The ignition temperature is 300 ° C. The substance therefore falls into temperature class T3.
use
3-methylpentane is used as a thinner for quick-drying paints, printing inks and adhesives. It is also used in the fuel, fuel and lubricant sectors. The compound is also used as a reference substance in spectroscopy and chromatography .
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on 3-methylpentane in the GESTIS substance database of the IFA , accessed on April 11, 2018(JavaScript required) .
- ↑ 3-Methylpentane data sheet from Sigma-Aldrich , accessed on May 18, 2017 ( PDF ).
- ↑ a b entry on methylpentane. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
- ↑ AA O'Kelly TO Sachanen: Alkylation of paraffin in the Presence of Homogeneous Catalysts. synthesis of Neohexane and Triptane , in: Ind. Eng. Chem. , 1946, 38 , pp. 462-467, doi : 10.1021 / ie50437a010 .
- ↑ CB Williamham, WJ Taylor, JM Pignocco, FD Rossini: Vapor Pressures and Boiling Points of Some Paraffin, Alkylcyclopentane, Alkylcyclohexane, and Alkylbenzene Hydrocarbons , in: J. Res. Natl. Bur. Stand. (US) , 1945, 35 , pp. 219-244.
- ↑ EJ Prosen, FD Rossini: Heats of combustion and formation of the paraffin hydrocarbons at 25 ° C , in: J. Res. Natl. Bur. Stand. (US) , 1945, 35 , pp. 263-267.
- ↑ a b c d e H. L. Finke, JF Messerly: 3-Methylpentane and 3-methylheptane: low-temperature thermodynamic properties , in: J. Chem. Thermodyn. , 1973, 5 , pp. 247-257, doi : 10.1016 / S0021-9614 (73) 80085-0 .
- ↑ K. Ohnishi, I. Fujihara, S. Murakami: Thermodynamic properties of decalins mixed with hexane isomers at 298.15K. 1. Excess enthalpies and excess isobaric heat capacities , in: Fluid Phase Equilib. , 1989, 46 , pp. 59-72, doi : 10.1016 / 0378-3812 (89) 80275-4 .
- ^ A b V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation , Blackwell Scientific Publications, Oxford, 1985, p. 300.
- ↑ I. Wadso: A heat of vaporization calorimeter for work at 25 ° C and for small Amounts of substances in: . Acta Chem Scand. , 1966, 20 , pp. 536-543, doi : 10.3891 / acta.chem.scand.20-0536 .
- ↑ a b c d T. E. Daubert: Vapor-Liquid Critical Properties of Elements and Compounds. 5. Branched Alkanes and Cycloalkanes , in: J. Chem. Eng. Data , 1996, 41 , pp. 365-372, doi : 10.1021 / je9501548 .
- ^ A b c E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ 3-methylpentane (Enius)





