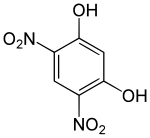
4,6-dinitroresorcinol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4,6-dinitroresorcinol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 4 N 2 O 6 | ||||||||||||||||||
| Brief description |
yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 200.11 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
215 ° C |
||||||||||||||||||
| pK s value |
3.98 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4,6-Dinitroresorcinol is a yellow solid that belongs to the group of phenols as well as to the group of nitroaromatics.
presentation
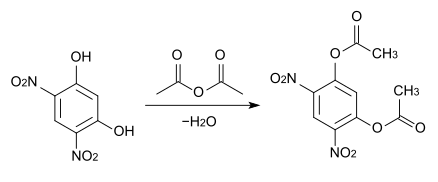
The preparation of 4,6-dinitroresorcinol is based on sterically hindering position 2 on the benzene nucleus before the actual nitration. For this, resorcinol is esterified with acetic anhydride to form the diacetate, then nitrated with nitric acid and sulfuric acid and the ester saponified.
Another synthetic route starts from 2-chlororesorcinol, which is first nitrated to 2-chloro-4,6-dinitroresorcinol. Then the chlorine atom is removed again.
Derivatives
The alkylation of 4,6-dinitroresorcinol leads to different ethers, the melting points of which are listed in the table below.
| connection | Monomethyl ether | Dimethyl ether | Monoethyl ether | Diethyl ether |
|---|---|---|---|---|
| CAS number | 51652-35-8 | 1210-96-4 | ||
| molar mass | 214.1 | 228.1 | 228.1 | 256.2 |
| Melting point | 113 ° C | 157 ° C | 77 ° C | 133 ° C |
Esterification with acetic anhydride leads to the diacetate, the melting point of which is 139 ° C.
use
4,6-Dinitroresorcinol is an intermediate in the manufacture of poly (p-phenylenebenzobisoxazole) (PBO).
Individual evidence
- ↑ Jared Ledgard: The preparatory manual of explosives , Third Edition, p. 428 ( limited preview in Google Book Search).
- ↑ a b c d J. Buckingham: Dictionary of organic compounds , Volume 9, p. 2768 ( limited preview in the Google book search).
- ↑ There is not yet a harmonized classification for this substance . A labeling of 4,6-dinitroresorcinol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on January 14, 2020, is reproduced from a self-classification by the distributor .
- ↑ Patent US4745232 : Process for preparing 4,6-dinitroresorcinol. Published May 17, 1988 , Inventors: ROBERT J. SCHMITT, DAVID S. ROSS, JAMES F. WOLFE.
- ↑ RJ Schmitt, DS Ross, JR Hardee, JF Wolfe: Synthesis of 4,6-Dinitroresorcinol , in: J. Org. Chem. , 1988, 53 (23) , pp. 5568-5569; doi : 10.1021 / jo00258a039 .
- ↑ Raj B. Durairaj: resorcinol: chemistry, technology, and applications , Birkhauser, 2005, ISBN 978-3-540-25142-2 , page 396 ( limited preview in Google Book Search).
- ↑ Raj B. Durairaj: resorcinol: chemistry, technology, and applications, Birkhauser, 2005, ISBN 978-3-540-25142-2 , page 106 ( limited preview in Google Book Search).