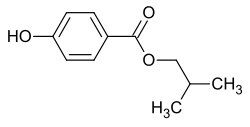
Isobutyl 4-hydroxybenzoate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Isobutyl 4-hydroxybenzoate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 11 H 14 O 3 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 194.23 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
74-78 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4-Hydroxybenzoic acid isobutyl ester is a chemical compound from the group of parabens . It is the ester of isobutyl alcohol and 4-hydroxybenzoic acid .
Extraction and presentation
The compound can be obtained by reacting 4-hydroxybenzoic acid and 2-methylpropan-1-ol .
properties
Isobutyl 4-hydroxybenzoate is a white solid that is practically insoluble in water.
use
4-Hydroxybenzoic acid isobutyl ester can be used for the synthesis of lipophilic alkyl parabens and as an additive for food and flavorings. It is also widely used as a preservative in cosmetic products. In the EU, use has not been permitted in food or cosmetics since 2014. The reason is the estrogenic effect.
Individual evidence
- ↑ a b c d e data sheet isobutyl 4-hydroxybenzoate, 98% from AlfaAesar, accessed on December 14, 2019 ( PDF )(JavaScript required) .
- ↑ a b c data sheet isobutyl 4-hydroxybenzoate, 97% from Sigma-Aldrich , accessed on December 14, 2019 ( PDF ).
- ↑ a b Michael Ash: Handbook of Preservatives . Synapse Info Resources, 2004, ISBN 978-1-890595-66-1 , pp. 424 ( limited preview in Google Book search).
- ↑ ChemSink: Synthesis of isobutyl 4-hydroxybenzoate via Ester Formation from Carboxylic Acid , accessed on December 14, 2019.
- ^ Federal Environmental Specimen Bank : isobutyl paraben , accessed on December 14, 2019.
- ↑ PD Darbre, JR Byford, LE Shaw, RA Horton, GS Pope, MJ Sauer: Oestrogenic activity of isobutylparaben in vitro and in vivo. In: J. Appl. Toxicol. 22, pp. 219-226, PMID 12210538 .

