4-trifluoromethoxyaniline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
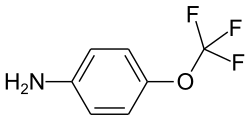
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4-trifluoromethoxyaniline | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 6 F 3 NO | ||||||||||||||||||
| Brief description |
yellow liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 177.12 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.32 g cm −3 (20 ° C) |
||||||||||||||||||
| boiling point |
73–75 ° C (13 hPa) |
||||||||||||||||||
| Refractive index |
1.463 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
4-Trifluoromethoxyaniline is a chemical compound of fluorine from the group of aniline derivatives .
Extraction and presentation
4-Trifluoromethoxyaniline can be obtained from the corresponding amide by Hofmann's amide degradation .
properties
4-trifluoromethoxyaniline is a yellow liquid.
use
4-Trifluoromethoxyaniline can be used as an intermediate in the manufacture of pharmaceuticals and agrochemicals.
literature
- M. Tugnait, EM Lenz, P. Phillips, M. Hofmann, M. Spraul, JC Lindon, JK Nicholson, ID Wilson: The metabolism of 4-trifluoromethoxyaniline and [13C] -4-trifluoromethoxyacetanilide in the rat: detection and identification of metabolites excreted in the urine by NMR and HPLC-NMR. In: J Pharm Biomed Anal. 28, 2002, pp. 875-885, PMID 12039629 .
Individual evidence
- ↑ a b c d e f g h i j data sheet 4- (Trifluoromethoxy) aniline, 98% from Sigma-Aldrich , accessed on June 20, 2018 ( PDF ).
- ↑ Andrei A. Gakh, Kenneth L. Kirk: Fluorinated Heterocycles . American Chemical Society, 2009, ISBN 978-0-8412-6953-8 , pp. 308 ( limited preview in Google Book search).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 1051 ( limited preview in Google Book search).


