Acifluorfen
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
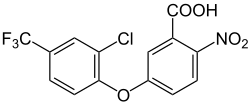
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Acifluorfen | |||||||||||||||||||||
| other names |
5- [2-chloro-4- (trifluoromethyl) phenoxy] -2-nitrobenzoic acid ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 14 H 7 ClF 3 NO 5 | |||||||||||||||||||||
| Brief description |
yellow-brown solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 361.66 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
151.5-157 ° C |
|||||||||||||||||||||
| solubility |
very heavy in water (120 mg l −1 at 23 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Acifluorfen is an active ingredient in crop protection and belongs to the class of phenol ether herbicides . It is a tan solid.
history
Acifluorfen was developed by Mobil Chem. Co. (now Bayer CropScience ) and Rohm & Haas (now BASF AG ) in the mid-1970s and launched on the market in 1980.
effect
Acifluorfen is a selective herbicide . It attacks as protoporphyrinogen - inhibitor in the photosynthesis of the treated plants and inhibits these. This is activated by the influence of light. After the photoelectric transport has been influenced, a membrane -liquid- oxidation follows , during which a lightening of the pigment can be observed. Since the damage caused by the inhibition of photosynthesis is severe, the treated plant dies.
use
It is mainly used against weeds and grass weeds in soybean, cotton and peanut cultivation. Acifluorfen is mostly used as the sodium salt .
Environmental aspects
Acifluorfen is not harmful to bees, but is poisonous for fish. The degradation takes place through photolytic decay with a half-life of 30 to 80 days.
proof
Residues can be determined in plants and soils using the GC or HPLC method .
Individual evidence
- ↑ a b c d e f g h Entry on Acifluorfen. In: Römpp Online . Georg Thieme Verlag, accessed on June 30, 2020.
- ↑ a b c d Entry on acifluorfen in the GESTIS substance database of the IFA , accessed on June 30, 2020(JavaScript required) .
- ↑ External identifiers or database links for acifluorfen sodium : CAS number: 62476-59-9, EC number: 263-560-7, ECHA InfoCard: 100.057.764 , GESTIS substance database : 149516 , PubChem : 44072 , ChemSpider : 40112 , Wikidata : Q27264899 .


