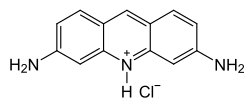
Acriflavinium chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Acriflavinium chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | Mixture of
|
|||||||||||||||
| Brief description |
reddish brown, crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | Mixture of substances | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
260 ° C (decomposition from 130 ° C) |
|||||||||||||||
| solubility |
good in water (300 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Acriflavinium chloride is a mixture of substances that is used as a medicinal substance ( antiseptic in the mouth and throat). It was patented by IG Farben in 1929.
From a chemical point of view, it is a mixture of about two thirds of 3,6-diamino-10-methylacridinium chloride (C 14 H 14 ClN 3 ) and about one third of 3,6-diaminoacridine hydrochloride (C 13 H 12 ClN 3 ), of which the former is a quaternary ammonium compound . Both compounds are derivatives of acridine .
effect
Acridine derivatives are effective against gram-positive bacteria . Acriflavinium chloride intercalates in nucleic acids and thereby inhibits the reproduction of bacteria and viruses . However, according to a study by the International Agency for Research on Cancer (IARC) , it may be carcinogenic .
Other uses
Acriflavinium chloride is also used for vital staining . In the past, 3,6-diamino-10-methylacridinium chloride was also used under the name trypaflavin as an agent against African trypanosomiasis ("sleeping sickness"), the pathogen of which is one of the trypanosomes .
Individual evidence
- ↑ a b c H. PT Ammon (Ed.): Hunnius. Pharmaceutical Dictionary . 9th edition. Walter de Gruyter, Berlin a. New York 2004.
- ↑ a b c data sheet Acriflavine hydrochloride from Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ↑ a b Data sheet acriflavinium chloride (PDF) from Merck , accessed on January 19, 2011.
- ↑ CJ Estler (Ed.): Pharmacology and Toxicology . 4th edition. Schattauer, Stuttgart a. New York 1995, p. 601.
- ↑ J. Falbe, M. Regitz (Ed.): Römpp Lexikon Chemie . 10th edition. Thieme, Stuttgart a. New York 1996-1999, p. 46.
- ↑ H. Beyer, W. Walter: Textbook of organic chemistry . 20th edition. Hirzel, Stuttgart 1984, p. 741.
