Diethyl adipate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
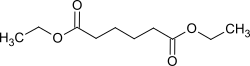
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Diethyl adipate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 18 O 4 | ||||||||||||||||||
| Brief description |
colorless and odorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 202.25 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.01 g cm −3 |
||||||||||||||||||
| Melting point |
−19.8 ° C |
||||||||||||||||||
| boiling point |
245 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.427 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Diethyl adipate is a chemical compound from the group of carboxylic acid esters .
Extraction and presentation
Diethyl adipate can be obtained by reacting adipic acid with ethanol in the presence of sulfuric acid or ammonium iron sulfate .
properties
Diethyl adipate is a flammable, hardly inflammable, colorless and odorless liquid that is practically insoluble in water.
use
Diethyl adipate is used as a plasticizer and intermediate for other chemical compounds (such as putrescine ).
Individual evidence
- ↑ a b c d e f g h i Entry on diethyl adipate in the GESTIS substance database of the IFA , accessed on December 15, 2018(JavaScript required) .
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 0-8493-0595-0 , pp. 180 ( limited preview in Google Book Search).
- ↑ Data sheet Diethyl adipate, ≥99%, FG from Sigma-Aldrich , accessed on December 15, 2018 ( PDF ).
- ↑ VK Ahluwalia, R. Aggarwal, VK Ahluwalia: Comprehensive Practical Organic Chemistry: Preparations And Quantitative Analysis . 2000, ISBN 81-7371-475-4 , pp. 79 ( limited preview in Google Book search).
- ↑ Zhao, R.-Q & Lin, J. (2000). Study on the catalytic synthesis of diethyl adipate with ammonium ferric sulfate . 20. 407-409.
- ↑ Entry on diethyl adipate in the Hazardous Substances Data Bank , accessed on December 15, 2018.