Aluminum ammonium sulfate dodecahydrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 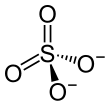 • 12 H 2 O • 12 H 2 O
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Aluminum ammonium sulfate dodecahydrate | |||||||||||||||
| other names |
Ammonium alum |
|||||||||||||||
| Molecular formula | NH 4 Al (SO 4 ) 2 • 12 H 2 O | |||||||||||||||
| Brief description |
colorless and odorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 453.33 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.64 g cm −3 |
|||||||||||||||
| Melting point |
93.5 ° C |
|||||||||||||||
| solubility |
150 g l −1 in water (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium Aluminum Sulfate , molecular formula: NH 4 Al (SO 4 ) 2 · 12 H 2 O, common name: ammonium alum, also as an ingredient of cosmetics as Ammonium Alum is designated, an ammonium - aluminum - double salt , the crystal water contains. It forms water-soluble, colorless cubic crystals with a relative molecular mass M r of 453.33, a density of 1.64 g / cm³ and a melting point of 93.5 ° C.
use
Ammonium alum is used in many ways, including in deodorants , the paper industry , tannery , for water purification or the production of caustic or flame retardants , in medicine as an astringent and in the cosmetics industry as a so-called " deocrystal ". In the food industry it is used as a firming agent and stabilizer . It is approved in the EU as a food additive under the designation E 523 with a permitted daily dose of one milligram of aluminum per kilogram of body weight.
See also
Individual evidence
- ↑ a b c d e Entry on ammonium aluminum sulfate in the GESTIS material database of the IFA , accessed on June 3, 2018(JavaScript required) .
- ↑ External identifiers or database links for aluminum ammonium sulfate anhydrate : CAS number: 7784-25-0, EC number: 232-055-3, ECHA InfoCard: 100.029.141 , GESTIS substance database : 4020 , PubChem : 24566 , ChemSpider : 2297489 , Wikidata : Q419370 .
- ^ Otto Helmboldt, L. Keith Hudson, Chanakya Misra, Karl Wefers, Wolfgang Heck: Aluminum Compounds, Inorganic . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH Verlag GmbH & Co. KGaA, 2000, ISBN 978-3-527-30673-2 , doi : 10.1002 / 14356007.a01_527.pub2 .

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)