Formic acid tert -butyl ester
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
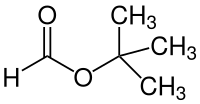
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Formic acid tert -butyl ester | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 5 H 10 O 2 | |||||||||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 102.13 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.879 g cm −3 |
|||||||||||||||||||||
| Melting point |
−94 ° C |
|||||||||||||||||||||
| boiling point |
82-83 ° C |
|||||||||||||||||||||
| Refractive index |
1.379 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Formic acid tert-butyl ester is a chemical compound from the group of carboxylic acid esters .
Extraction and presentation
Formic acid tert -butyl ester can be prepared by reaction of tert -butanol with formic acid in the presence of sulfuric acid are recovered. The compound is also formed when calcium formate is reacted with formic acid. It is one of the by-products of the breakdown of methyl tert-butyl ether (MTBE).
properties
Formic acid tert -butyl ester is a colorless liquid. In the environment the compound decomposes mainly by hydrolysis to tert -butyl alcohol and formic acid. The half-lives in aqueous solution are 6 hours at a pH value of 2 (at 4 ° C), 5 days at pH 7 (at 22 ° C) and 8 minutes at pH 11 (at 22 ° C).
use
Formic acid tert -butyl ester can be used in gasoline formulations.
Individual evidence
- ↑ a b c d e f g Entry on tert-butyl formate in the GESTIS substance database of the IFA , accessed on December 30, 2018(JavaScript required) .
- ↑ a b c EPA: Provisional Peer-Reviewed Toxicity Values for tert-butyl formats
- ↑ a b data sheet tert-butyl formats, 99% from Sigma-Aldrich , accessed on December 30, 2018 ( PDF ).
- ↑ Entry on tert-butyl formats in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 30, 2018. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Houben-Weyl Methods of Organic Chemistry Vol. E 5, 4th Edition Supplement Carboxylic Acids, Carboxylic Acid Derivatives . Georg Thieme Verlag, 2014, ISBN 3-13-181154-4 , p. 663 ( limited preview in Google Book search).
- ↑ Hans Günther Boit, Friedrich Konrad Beilstein, Friedrich Richter: Beilstein's Handbook of Organic Chemistry: Third and Fourth ... Springer, 1958, p. 318 ( limited preview in Google Book search).

