Ammonium fluorosulfonate
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ N 3− __ H + __ S 6+ __ O 2− __ F - | ||||||||||
| Crystal system | ||||||||||
| Space group |
Pnma (No. 62) |
|||||||||
| Lattice parameters |
|
|||||||||
| General | ||||||||||
| Surname | Ammonium fluorosulfonate | |||||||||
| other names |
Ammonium fluorosulfate |
|||||||||
| Ratio formula | NH 4 SO 3 F | |||||||||
| Brief description |
colorless crystals |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 117.10 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
245 ° C |
|||||||||
| solubility | ||||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Ammoniumfluorosulfonat , NH 4 SO 3 F is a chemical bond between the ammonium and the fluorosulfonate - anion .
Extraction and presentation
Ammonium fluorosulfonate is produced by the reaction of ammonium fluoride and sulfur trioxide .
properties
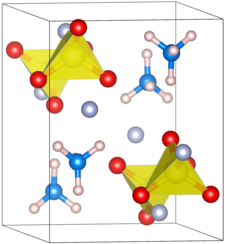
The ammonium salt of fluorosulfonic acid forms an orthorhombic crystal system . It has the space group Pnma (space group no. 62) . The lattice parameters are a = 8.972 Å , b = 5.996 Å, c = 7.542 Å and Z = 4. The distance between the sulfur and the oxygen is 1.45 Å. The length of the sulfur – fluorine bond is 1.55 Å. The angles of the O – S – O and F – S – O bonds are 113 ° and 106 °, respectively.
Individual evidence
- ↑ Dale L. Perry: Handbook of Inorganic Compounds . Ed .: Sidney L. Phillips. CRC Press, 1995, ISBN 0-8493-8671-3 , pp. 23 .
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics . CRC Press, 2016, ISBN 1-4987-5429-5 , pp. 4-46 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ K. Jones: The Chemistry of Nitrogen: Pergamon Texts in Inorganic Chemistry . Elsevier, 2013, ISBN 1-4831-8756-X , pp. 249 .
- ↑ K. O'Sullivan, RC Thompson, James Trotter: Crystal structure of ammonium fluorosulphate . J. Chem. Soc., 1970, pp. 1814–1817 , doi : 10.1039 / J19700001814 .
