Ammonium hexafluorosilicate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 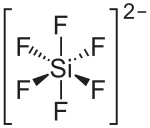
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium hexafluorosilicate | |||||||||||||||
| other names |
Ammonium hexafluorosilicate |
|||||||||||||||
| Molecular formula | (NH 4 ) 2 [SiF 6 ] | |||||||||||||||
| Brief description |
colorless to white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 178.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.01 g cm −3 |
|||||||||||||||
| solubility |
Easily soluble in water (186 g l −1 at 17 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium hexafluorosilicate is an inorganic chemical compound from the group of hexafluorosilicates .
Occurrence
Ammonium hexafluorosilicate occurs naturally in the form of the minerals bararite and cryptohalite .
Extraction and presentation
Ammonium hexafluorosilicate can be obtained by reacting hexafluorosilicic acid with ammonia .
properties
Ammonium hexafluorosilicate is a crystalline, colorless to white solid that is easily soluble in water. It decomposes when heated above 145 ° C, producing hydrogen fluoride and silicon tetrafluoride . Its aqueous solution is acidic. The compound comes in three modifications. A shape with a cubic crystal structure (cryptohalite shape) with the space group Fm 3 m (space group no. 225) , a shape with a trigonal crystal structure (bararite shape) with the space group P 3 m 1 (space group no. 164) and one Shape with hexagonal crystal structure with space group P 6 3 mc (space group no.186) . The cryptohalite form changes irreversibly into the bararite form under pressure.
use
Ammonium hexafluorosilicate is used as a disinfectant and analytical reagent. It is also used in the etching of glass, metal casting and electroplating and is used as a wood preservative and dirt protection agent in textiles. It also serves as a soldering flux and is also used to treat tooth decay without discolouring demineralized tooth enamel.
Individual evidence
- ↑ a b c d e f g Entry on ammonium hexafluorosilicate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Entry on alkalifluorosilicates (NH4) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 27, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on AMMONIUM SILICOFLUORIDE in the Hazardous Substances Data Bank , accessed on November 25, 2016.
- ↑ Pierre Villars, Karin Cenzual, Roman Gladyshevskii: Handbook . Walter de Gruyter GmbH & Co KG, 2015, ISBN 978-3-11-031174-7 , p. 1081 ( limited preview in Google Book search).
- ↑ Bodie Douglas, Shi-Ming Ho: Structure and Chemistry of Crystalline Solids . Springer Science & Business Media, 2007, ISBN 978-0-387-36687-6 , pp. 111 ( limited preview in Google Book search).
- ↑ Elena V. Boldyreva, Tatyana P. Shakhtshneider, Heidrun Sowa, Hans Ahsbahs: Effect of hydrostatic pressure up to 6 GPa on the crystal structures of ammonium and sodium hexafluorosilicates, (NH 4 ) 2 SiF 6 and Na 2 SiF 6 ; a phase transition in (NH 4 ) 2 SiF 6 at 0.2-0.3 GPa. In: Zeitschrift für Kristallographie , 2007, Volume 222, Issue 1, pp. 23-29 doi : 10.1524 / zkri.2007.222.1.23 .
- ↑ Data sheet Ammonium hexafluorosilicate, 99.999% (metals basis) from AlfaAesar, accessed on November 25, 2016 ( PDF )(JavaScript required) .
- ^ Y. Hosoya, E. Watanabe, K. Tadokoro, T. Inoue, M. Miyazaki, FR Tay: Effects of ammonium hexafluorosilicate application on demineralized enamel and dentin of primary teeth. In: Journal of oral science. Volume 54, Number 3, September 2012, pp. 267-272, PMID 23047038 .

![{\ displaystyle \ mathrm {2 \ NH_ {3} + H_ {2} [SiF_ {6}] \ longrightarrow (NH_ {4}) _ {2} [SiF_ {6}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8118ba86a4ad7f3edc7636674f146d68f8e3973f)