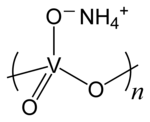
Ammonium metavanadate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium metavanadate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | NH 4 VO 3 | |||||||||||||||
| Brief description |
colorless odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 116.98 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.33 g cm −3 |
|||||||||||||||
| Melting point |
200 ° C (decomposition) |
|||||||||||||||
| solubility |
little in water:
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium metavanadate is a colorless and odorless solid from the group of vanadates .
Manufacturing
Ammonium metavanadate can be produced by an ion exchange reaction of sodium metavanadate with ammonium chloride :
properties
Ammonium metavandate is a solid, colorless in its pure state, which is poorly soluble in water, ethanol and ether , but readily soluble in dilute ammonia . The color changes to yellowish as soon as it contains more vanadium (V) oxide V 2 O 5 than corresponds to the stoichiometry. The yellowish colorations are derived from polyvanadates. Aqueous solutions of ammonium metavanadate react almost neutrally . The pH of a 5% solution at 20 ° C is 6.5.
Above 50 ° C, it loses ammonia and easily changes to vanadium (V) oxide when heated dry.
use
Ammonium vanadate is used for the production of catalysts for organic and inorganic syntheses, for the production of paints and varnishes as a drying agent for paintings and inks. It is used in food analysis for the photometric determination of phosphate .
See also
- Ammonium orthovanadate , (NH 4 ) 3 VO 4
- Ammonium hexavanadate , (NH 4 ) 2 V 6 O 16
Individual evidence
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1425.
- ↑ a b c d e f Entry on ammonium vanadate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c d Robert H. Baker et al .: Ammonium metavanadate . In: Ludwig F. Audrieth (Ed.): Inorganic Syntheses . tape 3 . McGraw-Hill, Inc., 1950, pp. 117-118 (English).
- ↑ a b Todini and Co spa: ammonium metavanadate. ( Memento of the original from January 7, 2016 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ L-06.00-9 Determination of the total phosphorus content in meat and meat products, 1992, Official Collection § 64 LFGB , Beuth-Verlag, Berlin.




